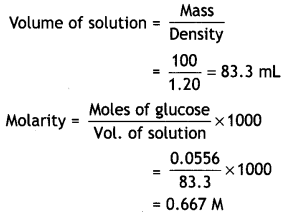
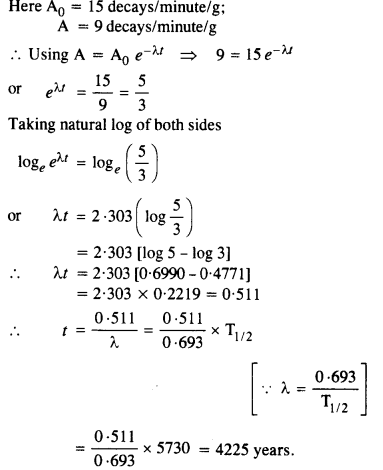
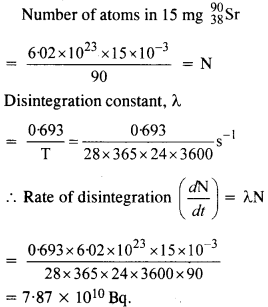
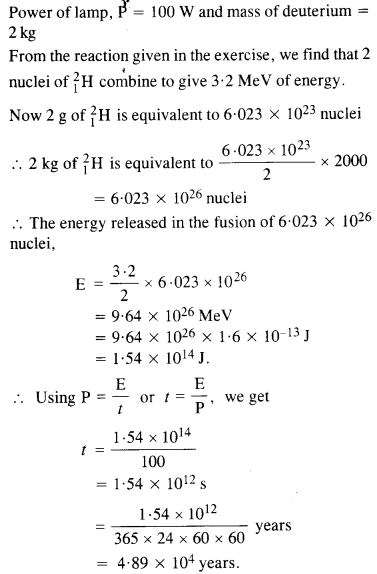
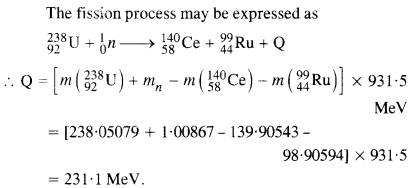
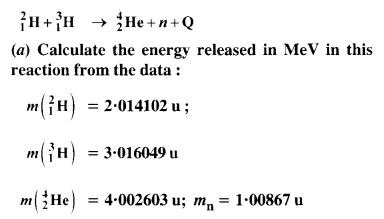
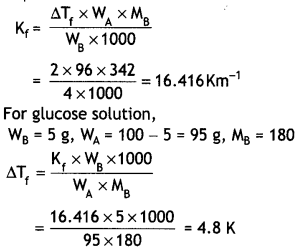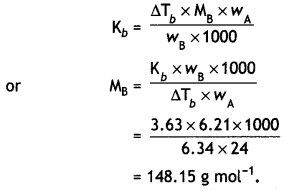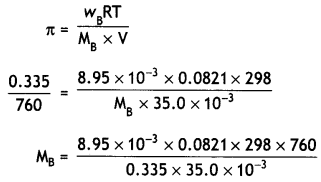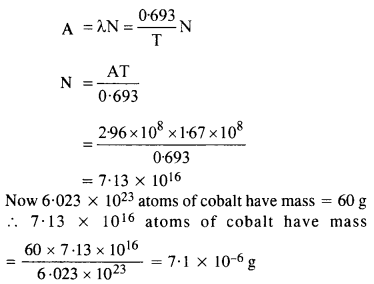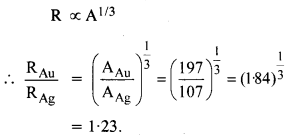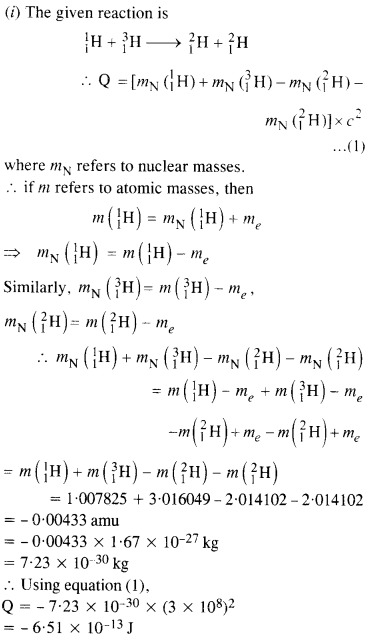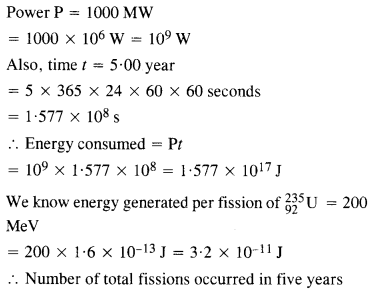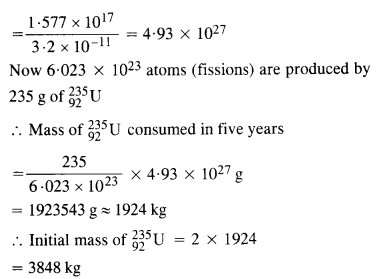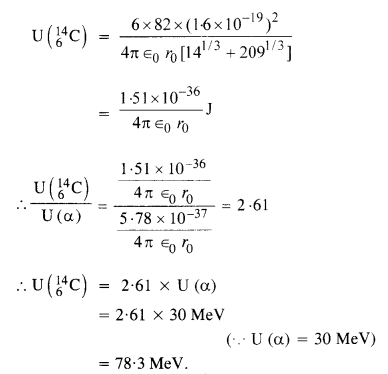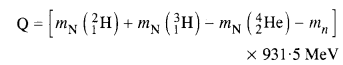NCERT Exemplar Solutions for Class 12 Biology chapter 8 Human Health and Diseases
These Solutions are part of NCERT Exemplar Solutions for Class 12 Biology. Here we have given NCERT Exemplar Solutions for Class 12 Biology chapter 8 Human Health and Diseases
Multiple Choice Questions
Question 1.
The term ‘Health’ is defined in many ways. The most accurate definition of the health would be
(a) health is the state of body and mind in a balanced condition
(b) health is the reflection of a smiling face
(c) health is a state of complete physical, mental and social well-being
(d) health is the symbol of economic prosperity.
Answer:
(c) : Health could be defined as a state of complete physical, mental and social well¬being. When people are healthy, they are more efficient at work. This increases productivity and brings economic prosperity. Health also increases longevity of people and reduces infant and maternal mortality.
Question 2.
The organisms which cause diseases in plants and animals are called
(a) pathogens
(b) vectors
(c) insects
(d) worms.
Answer:
(a) : A wide range of organisms belonging to bacteria, viruses, fungi, protozoans, helminths, etc. could cause diseases in pants and animals including humans. Such disease causing organisms are called pathogens. Most parasites are therefore pathogens as they cause harm to the host by Jiving in (or on) them. The pathogens can enter our body by various means, multiply and interfere with normal vital activities, resulting in morphological and functional damage.
Question 3.
The chemical test that is used for diagnosis of typhoid is
(a) ELISA-Test
(b) ESR – Test
(c) PCR – Test
(d) Widal-Test.
Answer:
(d) :Salmonella typhi is a pathogenic bacterium which causes typhoid fever in human beings. These pathogens generally enter the small intestine through food and water contaminated with them and migrate to other organs through blood. Sustained high fever (39 to 40°C), weakness, stomach pain, constipation, headache and loss of appetite are some of the common symptoms of the disease. Intestinal perforation and death many occur in severe cases. Typhoid fever could be confirmed by Widal test. Widal test is an agglutination test for the presence of antibodies against the Salmonella organisms that cause typhoid fever.
Question 4.
Diseases are broadly grouped into infectious and non-infectious diseases. In the list given below, identify the infectious diseases.
(1) Cancer
(2) Influenza
(3) Allergy
(4) Small pox
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (ii) and (iv)
Answer:
(d) : Diseases can be broadly grouped into infectious and non-infectious. Diseases which are easily transmitted from one person to another, are called infectious diseases. Influenza and small pox are infectious diseases which are spreaded by direct contact, inhalation and droplet infections. Cancer and allergy are non-communicable diseases, as these diseases remain confined to the person who suffer from them. They are not transmitted from infected person to a healthy person.
Question 5.
The sporozoites that cause infection, when a female Anopheles mosquito bites a person, are formed in
(a) liver of the person
(b) RBCs of mosquito
(c) salivary glands of mosquito
(d) intestine of mosquito.
Answer:
(d) : When a female Anopheles mosquito bites an infected person, gametocyte of Plasmodium enter the mosquito’s gut along with blood meal and fuse to form zygote which elongates and becomes motile, called ookinets. Ookinetes bore through the wall of gut and change to oocysts. Inside oocyst, sporozoites are formed. Mature infective stage(sporozoite) are released in the body cavity of mosquito and migrate to salivary gland of mosquito. When these mosquitoes bite a human, the sporozoites are introduced into his/her body, thereby initiating the disease in the host’s body.
Question 6.
The disease chikungunya is transmitted by
(a) houseflies
(b) Aedes mosquitoes
(c) cockroach
(d) female Anopheles.
Answer:
(b) : Chikungunya is caused by chikungunya virus. The virus was first isolated from human patients and Aedes aegypti mosquitoes from Tanzania in 1952. The disease is transmitted by the bite of Aedes aegypti mosquito.
Question 7.
Many diseases can be diagnosed by observing the symptoms in the patient. Which group of symptoms are indicative of pneumonia?
(a) Difficulty in respiration, fever, chills, cough, headache.
(b) Constipation, abdominal pain, cramps, blood clots.
(c) Nasal congestion and discharge, cough, sore throat, headache.
(d) High fever, weakness, stomach pain, loss of appetite and constipation.
Answer:
(a) : Bacteria like Streptococcus pneumoniae and Haemophilus influenzae are responsible for the disease pneumonia in humans which infects the alveoli (air filled sacs) of the lungs. As a result of the infection, the alveoli get filled with fluid leading to severe problems in respiration. The symptoms of pneumonia include fever, chills, cough and headache.
Question 8.
The genes causing cancer are
(a) structural genes
(b) expressor genes
(c) oncogenes
(d) regulatory genes.
Answer:
(c) : Cancer is one of the most serious medical problems caused by the abnormal cell growth and proliferation due to the loss of regulation. Cancer-causing genes are oncogenes. Oncogene results from mutation of a normal gene. It is capable of both initiation and continuation of malignant transformation of normal cells.
Question 9.
In malignant tumours, the cells proliferate, grow rapidly and move to other parts of the body to form new tumours. This stage of disease is called
(a) metagenesis
(b) metastasis
(c) teratogenesis
(d) mitosis.
Answer:
(b) : There are two major types of tumours with respect to overall form or growth pattern. If the tumour cells remain in place to form a compact mass, the tumour is benign. In contrast, cells from malignant or cancerous tumours can actively spread, throughout the body in a process known as metastasis, often by floating in the blood and establishing secondary tumours.
Question 10.
When an apparently healthy person is diagnosed as unhealthy by a psychiatrist, the reason could be that
(a) the patient was not efficient at his work
(b) the patient was not economically pros-perous
(c) the patient shows behavioural and social maladjustment
(d) he does not take interest in sports.
Answer:
(c) : If a patient shows behavioural and social maladjustment then, he cannot be considered as healthy. Health is the condition in which the organism (and all of its parts) performs its vital functions normally. It is a state of physical, social and mental well-being and not merely the absence of disease.
Question 11.
Which of the following are the reason(s) for rheumatoid arthritis? Choose the correct option.
(i) The ability to differentiate pathogens or foreign molecules from self cells increases.
(ii) Body attacks self cells.
(iii) More antibodies are produced in the body.
(iv) The ability to differentiate pathogens or foreign molecules from self cells is lost.
(a) (i)and(ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(iv) (d) (i) and (iii)
Answer:
(b) : Rheumatoid arthritis is a disorder of the body’s defence mechanisms in which an immune response is elicited against its own tissues, which are thereby damaged or destroyed i.e., autoimmune disease.
Rheumatoid arthritis is the second most common form of arthritis (after osteoarthritis). It typically involves the joints of the fingers,
wrists, feet, and ankles, with later involvement of the hips, knees, shoulders, and neck. It is a disease of the synovial lining of joints; the joints are initially painful, swollen, and stiff and are usually affected symmetrically. As the disease progresses the ligaments supporting the joints are damaged and there is erosion of the bone, leading to deformity of the joints. Tendon sheath can be affected, leading to tendon rupture.
Question 12.
AIDS is caused by HIV. Among the following, which one is not a mode of transmission of HIV?
(a) Transfusion of contaminated blood.
(b) Sharing the infected needles.
(c) Shaking hands with infected persons.
(d) Sexual contact with infected persons.
Answer:
(c) : AIDS is a result of an infection by the human immunodeficiency virus (HIV) :
HIV can be transmitted by the following ways.
- Transfusion of infected blood.
- Use of contaminated needles and syringes to inject drugs or vaccines.
- Use of contaminated razors.
- Use of contaminated needles for boring pinnae.
- Sexual intercourse with an infected partner without a condom.
- From infected mother to child through placenta.
- Artificial insemination.
- Organ transplantation.
Question 13.
‘Smack’is a drug obtained from the
(a) latex of Papaver somniferum
(b) leaves of Cannabis sativa
(c) flowers of Datura
(d) fruits of Erythroxylum coca.
Answer:
(a) : Heroin commonly called smack is chemically diacetylmorphine which is a white, odourless, bitter crystalline compound. This is obtained by acetylation of morphine, which is extracted from the latex of poppy plant (Papaver somniferum).
Question 14.
The substance produced by a cell in viral infection that can protect other cells from further infection is
(a) serotonin
(b) colostrum
(c) interferon
(d) histamine.
Answer:
(c) : Interferons are glycoproteins released by living cells in response to viral attack, and make the surrounding cells resistant to viral infection by inhibiting multiplication of viral particles.
Interferons are divided into three groups based on the cell of origin, namely leucocyte (alpha interferon), fibroblast (beta interferon) and lymphocyte (gamma interferon). They are cytokine (chemical mediators) barriers.
Question 15.
Transplantation of tissues/organs to save certain patients often fails due to rejection of such tissues/organs by the patient. Which type of immune response is responsible for such rejections?
(a) Auto immune response
(b) Humoral immune response
(c) Physiological immune response
(d) Cell-mediated immune response
Answer:
(d) : Cell mediated immune response is responsible for graft rejection. Tissue matching and blood group matching are essential before undertaking any graft/transplant and even after this the patient has to take immuno¬suppressants throughout his/her life as body is able to differentiate ‘self’ from ‘non-self’.
Question 16.
Antibodies present in colostrum which protect the new born from certain diseases is of
(a) IgGtype
(b) IgA type
(c) IgDtype
(d) IgEtype.
Answer:
(b) : IgA antibody is the major antibody present in the colostrum. It protects the infant from inhaled and ingested pathogens. It is the second most abundant class of immunoglobulins, constituting about 13% of serum immunoglobulins.
Question 17.
Tobacco consumption is known to stimulate secretion of adrenaline and nor-adrenaline. The component causing this could be
(a) nicotine
(b) tannic acid
(c) curaimin
(d) catechin.
Answer:
(a) : Tobacco contains a large number of chemical substances including nicotine, an alkaloid. Nicotine stimulates adrenal gland to release adrenaline and nor-adrenaline into blood circulation, both of which raise blood pressure and increase heart rate.
Question 18.
Anti venom against snake poison contains
(a) antigens
(b) antigen-antibody complexes
(c) antibodies
(d) enzymes.
Answer:
(c) : In case of snakebite, the injection which is given to a patient, contains preformed antibodies against the snakevenom. This type of immunisation is called passive immunisation.
Question 19.
Which of the following is not a lymphoid tissue?
(a) Spleen
(b) Tonsils
(c) Pancreas
(d) Thymus
Answer:
(c) : Lymphoid organs are the organs where origin and/or maturation
and proliferation of lymphocytes occur. The primary lymphoid organs are bone marrow and thymus, where immature lymphocytes differentiate into antigen – sensitive lymphocytes. After maturation the lymphocytes migrate to secondary lymphoid organs like spleen, lymph nodes, tonsils, Peyer’s patches of small intestine and mucosa associated lymphoid tissue (MALT). The secondary lymphoid organs provide the sites for interaction of lymphocytes with the antigen, which then proliferate to become effector cells. Pancreas is not a lymphoid tissue.
Question 20.
Which of the following glands is large sized at birth but reduces in size with ageing?
(a) Pineal
(b) Pituitary
(c) Thymus
(d) Thyroid _____
Answer:
(c) : The thymus is a lobed organ located near the heart and beneath the breastbone. The thymus is quite large at the time of birth but keeps reducing in size with age and by the time puberty is attained, it reduces to a very small size.
Question 21.
Haemozoin is a
(a) precursor of haemoglobin
(b) toxin released from Streptococcus infected cells.
(c) toxin released from Plasmodium infected cells.
(d) toxin released from Haemophilus infected ‘cells.
Answer:
(c) : Plasmodium enters the human body as sporozoites (infectious form) through the bite of infected female Anopheles mosquito. The parasites initially multiply within the liver cells and then attack the red blood cell (RBCs) resulting in their rupture. The rupture of RBCs is associated with release of a toxic substance, haemozoin, which is responsible for the chill and high fever recurring every three to four days.
Question 22.
One of the following is not the causal organism for ringworm.
(a) Microsporum
(b) Trichophyton
(c) Epidermophyton
(d) Macrosporum
Answer:
(d) : Many fungi belonging to the genera Microsporum, Trichophyton and Epidermophyton are responsible for ringworms which is one of the most common infectious diseases in man. Appearance of dry, scaly lesions on various parts of the body such as skin, nails and scalp are the main symptoms of the disease.
Question 23.
A person with sickle cell anaemia is
(a) more prone to malaria
(b) more prone to typhoid
(c) less prone to malaria
(d) less prone to typhoid.
Answer:
(c) : Sickle cell trait protects against malaria. Malarial parasite is unable to penetrate the erythrocyte membrane of sickle shaped erythrocytes, reducing the number of parasites that actually infect the host thus conferring some protection against the disease.
Very Short Answer Type Questions
Question 1.
Certain pathogens are tissue/organ specific. Justify the statement with suitable examples.
Answer:
Some pathogens attack specific organs or tissues, such as :
- Vibrio cholerae – It is a bacterium which causes cholera. It attacks digestive tracts and intestine and spreads through contaminated food and drinks.
- Streptococcus pneumoniae – It is a bacterium which causes pneumonia. It is a serious disease of lungs characterised by
accumulation of mucus/fluid in alveoli and bronchioles hence, breathing becomes difficult.
Question 2.
The immune system of a person is suppressed. In the ELISA test, he was found positive to a pathogen.
(a) Name the disease the patient is suffering from.
(b) What is the causative organism?
(c) Which cells of body are affected by the pathogen?
Answer:
(a) The patient must be suffering from Acquired Immune Deficiency Syndrome (AIDS). .
(b) The causative agent is Human Immuno-deficiency Virus (HIV).
(c) Virus affects helper T cells of the body.
Question 3.
Where are B-cells and T-cells formed? How do they differ from each other?
Answer:
B-cells and T-cells are lymphocytes which are responsible for immune response in the body. Both B-cells and T-cells are formed in bone marrow.
Differences between B-cells and T-cells are
| B-cells | T-cells |
| (1) Bone marrow is the site of both formation and maturation. | They are produced in bone marrow but mature in thymus. |
| (2) B-cells are responsible for humoral or antibody mediated immunity. | T-cells are responsible for cell mediated immunity. |
Question 4.
Given below are the pairs of pathogens and the diseases caused by them. Which out of these is not a matching pair and why?
(a) Virus common cold
(b) Salmonella typhoid
(c) Microsporum filariasis
(d) Plasmodium malaria
Answer:
(c) : Microsporum is a fungus and causes ringworm. It attacks the hair and skin.Filariasis is caused by Wuchereria bancrofti, a helmirith, which results in swelling of limbs, scrotal sacs and breasts.
Question 5.
What would happen to immune system, if thymus gland is removed from the body of a person?
Answer:
T-lymphocytes get differentiated in thymus gland and are responsible for cell mediated immunity (CMI). If thymus gland is removed, then CMI will weaken and person will become more susceptible to infectious diseases.
Question 6.
Many microbial pathogens enter the gut of humans along with food. What are the preventive barriers to protect the body from such pathogens? What type of immunity do you observe in this case?
Answer:
Physiological barriers of innate immunity protect the body against the pathogens which enter the gut along with food. These barriers are as follows:
- Saliva contains lysozyme which kills the microorganisms that are not the normal inhabitants of the buccal cavity and come with food and drinks.
- Bile, a bitter alkaline secretion of the liver, checks the growth of foreign bacteria on semidigested food, the chyme, in the intestine.
- Acidity of gastric juice kills most of the microorganisms entering the body through digestive tract.
Question 7.
Why is mother’s milk considered the most appropriate food for a new born infant?
Answer:
Mother’s milk (colostrum) contains all the necessary nutrients needed by the newborn and has abundant antibodies (IgA) which provide passive immunity and protect the infant for few months after birth.
Question 8.
What are interferons? How do interferons check infection of new cells?
Answer:
Interferons are cytokine barriers of innate immunity. These are proteins secreted by virus infected cells which protect non- infected cells from further viral infections.
Question 9.
In the figure, structure of an antibody molecule is shown. Name the parts A, B and C
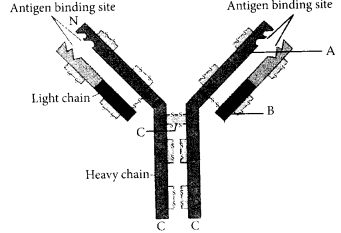
Answer:
A – Variable region of heayy chain
B – Constant region of light chain .
C – Disulphide bond
Question 10.
If a regular dose of drug or alcohol is not provided to an addicted person, he shows some withdrawal symptoms. List any four such withdrawal symptoms.
Answer:
Withdrawal symptoms shown by an addicted person when not provided with drug or alcohol includes anxiety, nausea, muscle twitch, sweating, nervousness and epilepsy etc.
Question 11.
Why is it that during changing weather, one is advised to avoid closed, crowded and airconditioned places like cinema halls etc.?
Answer:
It is advised to avoid overcrowded places during changing weather because chances of getting infections are very high during this time. This is because changing seasons are the time when infectious agents are more prevalent as moist condition favours pathogen growth. Moreover, people are more vulnerable as their body system is busy in adapting to the changing environmental conditions.
Question 12.
The harmful allele of sickle cell anaemia has not been eliminated from human population. Such afflicted people derive some other benefit. Discuss.
Answer:
Sickle cell anaemia is caused due to a recessive allele in haemoglobin. It is lethal
in homozygous (HbsHbs) condition and the person dies in early age but in heterozygous condition (HbAHbs) person is carrier of disease. Despite having harmful effect, the allele for sickle-cell anaemia continues to persist in human population because it has survival value in malaria infested areas like tropical Africa. Heterozygous individuals show resistance to malarial infection, because malarial parasite is unable to penetrate the membrane of sickle shaped erythrocytes.
Question 13.
Lymph nodes are secondary lymphoid organs. Explain the role of lymph nodes in our immune response.
Answer:
The lymph nodes are small solid structures located at different points along the lymphatic system. Lymph nodes serve to trap the microorganisms or other antigens, which happen to get into the lymph and tissue fluid. Antigens trapped in the lymph nodes are responsible for the activation of lymphocytes present there and cause the immune response.
Question 14.
Why is an antibody molecule represented as H2L2?
Answer:
Antibody molecule is made up of four polypeptide chains-2 Heavy (H) and 2 Light (L), therefore it is represented as H:L2.
Question 15.
What does the term ‘memory’ of the immune system mean?
Answer:
Memory response is a feature of acquired immunity. It develops during first encounter between specific foreign agent and body’s immune system. Second encounter with the same pathogen generates quicker and heightened immune response due to activated memory cells.
Question 16.
lf a patient is advised anti retroviral therapy, which infection is he suffering from? Name the causative organism.
Answer:
The patient must be suffering from Required Immune Deficiency Syndrome (AIDS) caused by Human Immunodeficiency Virus (HIV), which is a member of retrovirus group
short Answer Type Questions
Question 1.
Differentiate between active immunity and passive immunity.
Answer:
Differences between active immunity and passive immunity are as follows:
| Active immunity | Passive immunity |
| (1) It develops in response to vaccine or infection. | It develops when readymade antibodies are injected from outside. |
| (2) It is long lasting. | It lasts for short period only. |
| (3) Antibodies are produced within the body. | Antibodies are injected from outside. |
| (4) It has no side effects. | It may cause reaction. |
| (5) Immunity is not immediate. A time lapse occurs for its development. | Immunity develops immediately |
Question 2.
Differentiate between benign tumour and malignant tumour.
Answer:
Difference between benign tumour and malignant tumour are as follows:
| Benign tumour | Malignant |
| (1) It is non-cancerous tumour | It is cancerous tumour. |
| (2) It does not show metastasis and remains confined to the affected organ. | It shows metastasis and spreads to other organs of the body. |
| (3) Rate of growth is slow. It shows definite growth. | Rate of growth is rapid. It shows indefinite growth. |
Question 3.
Do you consider passive smoking is more dangerous than active smoking? Why?
Answer:
Passive smoking is inhalation of air containing tobacco smoke exhaled by an active smoker. Passive smoker is at higher risk as compared to an active smoker, because active smoker might inhale 10% of the smoke, but passive smoker inhales 90% of the smoke.
Question 4.
“Prevention is better than cure”. Comment.
Answer:
Prevention is always better than cure.This is because there are some diseases which have detrimental effects on our body and lives. Certain diseases like cancer and AIDS etc., are fatal. They can be avoided if our health systems focus on prevention of such diseases rather than concentrating on treatment methods. Many people can be saved from contracting diseases. Similar conditions apply for communicable, infectious diseases. If preventive measures are undertaken, their spread, can be checked and controlled easily.
Question 5.
Explain any three preventive measures to control microbial infections.
Answer:
Maintenance of personal and public hygiene is important to control microbial infections. Some of the measures that can be undertaken are as follows:
- Eradication of vectors and destroying their breeding sites
- Use of mosquito nets and repellents.
- Proper disposal of waste.
- Periodic cleaning of water reservoirs.
- Checking water stagnation and garbage accumulation.
- Implementation of vaccination and immunisation programmes for diseases.
Question 6.
In the given flow diagram, the replication of retrovirus in a host is shown. Observe and answer the following questions.
a. Fill in (1) and (2).
b. Why is the virus called retrovirus?
c. Can the infected cell survive while viruses are being replicated and released?
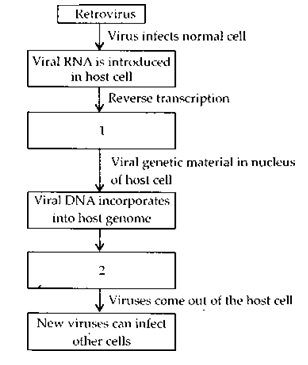
Answer:
(a)
(1) – Viral DN A is produced by reverse transcriptase.
(2) – New viral RNA is produced by the infected cell.
(b) HIV is called retrovirus because viral DNA is produced from viral RNA by reverse transcription.
(c) Infected cell survives but T-lymphocytes decrease in number due to replication and release of virus.
Question 7.
“Maintenance of personal and public hygiene is necessary for prevention and control of many infectious diseases”. Justify the statement giving suitable examples.
Answer:
Maintenance of personal and public hygiene is very important for prevention and control of many infectious diseases because diseases spread through contaminated food, water, air and through vectors. Measures for personal hygiene include keeping the body clean, consumption of clean drinking water, food, etc.
Public hygiene includes proper disposal of waste and excreta, periodic cleaning and disinfection of water reservoirs, pools, and tanks and observing standard practices of hygiene in public catering.
In case of air-borne diseases such as pneumonia and common cold, close contact with the infected persons or their belongings should be avoided. Vector borne diseases can be controlled by eliminating the vectors and their breeding places. This can be achieved by avoiding stagnation of water in and around residential areas, regular cleaning of household coolers, use of mosquito nets, etc.
Question 8.
The following table shows certain diseases, their causative organisms and symptoms. Fill the gaps.”
| Name of the Disease | Causative organism | Symptoms |
| (1) Ascariasis | Ascaris | — |
| (2) — | Trichophyton | Appearance of dry, scaly lesions on various parts of the body |
| (3) Typhoid | High fever, weakness, headache, stomach pain, constipation | |
| (4) Pneumonia | Streptococcus pneumoniae | — |
| (5) | Rhino viruses | Nasal congestion and discharge, sorethroat, cough, headache |
| (6) Filariasis | — | Inflammation in lower limbs |
Answer:
(i) Abdominal pain, indigestion, nausea, vomiting etc.
(ii) Ringworm
(iii) Salmonella typhi
(iv) Difficulty in breathing, fever, cough, headache, chills, lips and finger nails may turn grey to bluish in colour
(v) Common cold
(vi) Wuchereria bancrofti
Question 9.
The outline structure of a drug is given below.
a. Which group of drugs does this represent?
b. What are the modes of consumption of these drugs?
c. Name the organ of the body which is affected by consumption of these drugs.
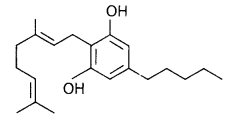
Answer:
(a) Cannabinoids
(b) Cannabinoids are generally taken by inhalation and oral ingestion.
(c) They affect cardiovascular system of the body.
Question 10.
Give the full form of CT and MRI. How are they different from each other?
Answer:
CT (Computed Tomography) is recon-struction of three dimensional image made by X rays directly on a computer instead of a photographic film. It is radiological invasive technique. It is used to diagnose diseases of brain, spinal cord, chest and abdomen and detection of tumours. MRI (Magnetic Resonance Imaging) is a non invasive, non radiological technique in which the image is reconstructed on computer by detecting MRI signals generated by nuclei of hydrogen atoms in a magnetic field using non-ionising radiations. It is used to obtain multiplanar imaging of soft living tissues like in case of tumours, muscular disorders, cardiovascular disorders, haemorrhage with superior resolution.
Question 11.
Many secondary metabolites of plants have medicinal properties. It is their misuse that creates problems. Justify the statement with an example.
Answer:
Drugs like barbiturates, amphetamines, benzodiazepines, lysergic acid diethylamides (LSD) and other similar drugs, that are normally used as medicines to help patients coping with mental illnesses like depression and insomnia are secondary metabolites of plants. Misuse of plant metabolites can impair one’s .physical, physiological or functional behaviour creating problem for the society. For example, Cocaine is natural alkaloid obtained from leaves of plant Erythroxylum coca, native to South America. Cocaine has vasoconstrictor properties and therefore, is a good local anaesthetic. It is chewed, eaten or sniffed in its powdered form or taken as a drink. It is a powerful CNS stimulant (interferes with the transport of the neurotransmitter dopamine) and induces a sense of well being and pleasure and delays fatigue. Its overdose causes hallucinations, headache, insomnia, convulsions, etc.
Question 12.
Why cannabinoids are banned in sports and games?
Answer:
Cannabinoids may enhance the performance of some athletes. Smoking cannabis may decrease anxiety, fear, depression, tension, etc., and improve self confidence, relaxation, well-being and sleep, etc. These effects may allow the athletes to perform better, especially under pressure and may reduce stress, experienced before and during competition. The increase in ‘risk taking’ associated with cannabinoids may possibly improve training and performance giving athlete a competitive edge. Moreover, cannabinoids increase bronchodilation (which may improve oxygenation of tissue) and have analgesic (pain relieving) properties which could permit athletes to work during injury. Moreover, long term use of cannabinoids causes various hazards to the health of abuser. Therefore, use of cannabinoids by sportsperson is not justified, as it will lead to unfair competition. Hence, their use is banned in sports and games.
Question 13.
What is secondary metabolism?
Answer:
Secondary metabolism consists of metabolic pathways and products of metabolism that seem to have no direct function in growth and development of plants and are not absolutely required for their survival. Secondary metabolism is responsible for many bioactive compounds used medicinally.
Question 14.
Drugs and alcohol give short-term ‘high’ and long-term’damages’. Discuss.
Answer:
Curiosity, need for adventure and excitement, peer pressure, etc., constitute some common causes which motivate youngsters towards drug and alcohol abuse. Short term effects of drugs/alcohols are generally pleasing and relaxing which habituate the abuser for using these again and again. But long term effects of drugs or alcohol are devastating and detrimental. For example, alcohol when taken occasionally in small quantities, acts as sedative, analgesic and anaesthetic. It stimulates the secretion of gastric juices. But, if a person becomes addicted to it, he consumes high concentration alcohol frequently. Heavy drinking causes depression. Suicide attempt is much common in alcoholics than in the rest of society.
Sexual relationship is usually deteriorated because of erectile dysfunction or rejection by the partner.
Caffeine is a mild stimulant and taken as beverages- tea, coffee, cocoa and cola drinks. Caffeine is CNS stimulant and thus increases alertness and thought. It also acts as a cardiac and respiratory stimulant. It is a mild diuretic and also increases basal metabolic rate. Excessive use may cause anxiety, irritability, diarrhoea, irregular heart beat (arrhythmia) and decreased concentration. It also causes indigestion and disturbs pancreatic and renal functions. If taken empty stomach, it can cause stomach ulcers.
Question 15.
Diseases like dysentery, cholera, typhoid etc., are more common in over crowded human settlements. Why?
Answer:
Overcrowded areas are generally unhygienic. This is because public hygeine is not maintained properly. Water bodies in these areas are generally contaminated with disease causing microbes. Water stagnation and garbage accumulation is often not checked.
Diseases like dysentery, cholera, typhoid etc., are water borne infectious diseases and may easily spread in overcrowded human dwellings.
Question 16.
From which plant cannabinoids are obtained? Name any two cannabinoids. Which part of the body is effected by consuming these substances?
Answer:
Cannabinoids are obtained from leaves, resins and flowers of hemp plants e.g., Cannabis sativa. Examples of cannabinoids are bhang, ganga, charas etc. They interact with receptors present in the brain and affect cardiovascular system of the body.
Question 17.
In the metropolitan cities of India, many children are suffering from allergy/asthma. What are the main causes of this problem. Give some symptoms of allergic reactions.
Answer:
Children living in metropolitan cities are more prone to allergies/asthma, mainly due to life style and increased level of pollution. Suspended particulate matter released by vehicles add more to the increasing pollution levels. Moreover children living in metropolitan cities are overprotected in their early childhood days and are more sensitive to allergens.
Allergy is hypersensitive response of the body against foreign particles (allergens). Common symptoms are sneezing, watery eyes, running nose, difficulty in breathing, irritation of throat, trachea and skin etc. Asthma related problems worsen due to changing weather conditions and increased pollution levels.
Question 18.
What is the basic principle of vaccination?How do vaccines prevent microbial infections? Name the organism from which hepatitis B vaccine is produced.
Answer:
Vaccine is a preparation of antigenic proteins or inactivated/live but weakened germs of a disease which on inoculation into a healthy person provides temporary or permanent, active immunity by inducing antibody formation.
Vaccination is based on the property of ‘memory’ of the immune system. When a vaccine is injected, it generates primary immune response of low intensity and also produces B cells and T cells. When vaccinated person is again attacked by same pathogen, the existing memory T and B cells recognise the antigen quickly and attack the invaders with a massive production of lymphocytes and antibodies. Hepatitis B vaccine is second generation vaccine that has been produced from yeast by recombinant DNA technology.
Question 19.
What is cancer? How is a cancer cell different from the normal cell? How do normal cells attain cancerous nature?
Answer:
Cancer is an abnormal and uncontrolled division of cells, that invade and destroy surrounding tissues. Cancer cells differ from normal cells in the following ways:
- Cancer cells multiply in an uncontrolled manner.
- They do not exhibit property of contact inhibition.
- They show metastasis.
- They have indefinite life span.
Cancer causing agents known as carcinogens include physical, chemical and biological agents which activate the proto-oncogenes or cellular oncogenes to oncogenes and cause deviation in growth pattern leading to uncontrolled growth of cells. They alter the behaviour of normal cells and make them cancerous.
Question 20.
A person shows strong unusual hypersensitive reactions when exposed to certain substances present in the air. Identify the condition. Name the cells responsible for such reactions. What precaution should be taken to avoid such reactions.
Answer:
Hypersensitive reaction to foreign substances is known as allergy. Substances which cause allergic reactions are called allergens. The common allergens are dust, pollen, feathers, paint. In allergic chemicals called histamine and serotonin are released from mast cells. Allergy can be avoided by reducing an exposure to allergen and by taking drugs like antihistamines, adrenaline and steroids.
Question 21.
For an organ transplant, it is an advantage to have an identical twin. Why?
Answer:
For an organ transplantation, tissue matching(histocompatibility)andblood group compatibility of donor and recipient are very important. If they do not match, organ may be rejected. This is because, immune system recognises the protein in the transplanted tissue or organ as foreign and initiates cellular immunity. Chances of matching of tissue as well as blood group are very high if donor and recipient are identical twins, because of genetic similarity. Transplantation between identical twins is known as isograft.
Question 22.
What are lifestyle diseases? How are they caused? Name any two such diseases.
Answer:
Diseases which occur due to improper changes in the life style are known as lifestyle diseases. These diseases may be caused due to over-eating or crash dieting, lack of exercise, sedentary lifestyle, smoking, alcoholism, drug addiction etc. Hypertension and obesity are common lifestyle diseases.
Question 23.
If there are two pathogenic viruses, one with DNA and other with RNA, which would mutate faster? And why?
Answer:
Pathogenic virus with RNA as genetic material will mutate faster than the one with DNA as genetic material, because
- RNA is chemically and structurally unstable. Uracil present in RNA is less stable than thymine in DNA.
- RNA is highly reactive, labile and easily degradable.
Long Answer Type Questions
Question 1.
Represent schematically the life cycle of a malarial parasite.
Answer:
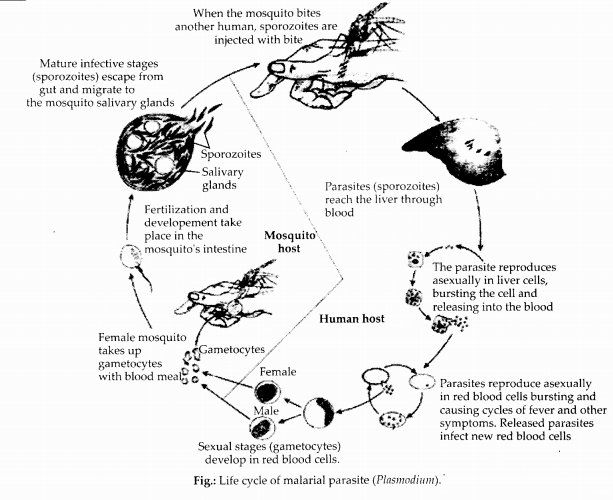
Question 2.
Compare the life style of people living in the urban areas with those of rural areas and briefly describe how the lifestyle affects their health.
Answer:
Lifestyle affects human health in various ways which are as follows:
(1) Food habits – People living in urban areas usually prefer fast food like pasta, pizza, burger, noodles etc., as compared to fresh, green leafy vegetables, fruits which are more consumed by villagers, because of this they suffer from obesity, hypertension and some cardiovascular problems.
(2) Residential areas — Due to lack of space in urban areas, residential areas are generally overcrowded. Sometimes there is no facility for proper disposal of garbage, no proper ventilation etc., therefore people become prone to food, water, and air borne diseases, like cholera, tuberculosis, typhoid, pneumonia etc. On the other hand, villages have clean air, human dwellings are not crowded and the environment is free of pollution. But due to lack of personal and public hygiene and malnutrition, villagers frequently suffer from various diseases.
Moreover, medical facilities in rural areas are not up to the mark. People of urban areas are exposed to good education, proper medical facilities etc.
(3) Exercise and sleep — Because of busy lifestyle, people in urban areas do not get enough rest and have no time for exercise. Whereas people in rural areas do a lot of physical work and have enough time to rest as well.
(4) Due to overprotected environment provided during early life, people in urban areas sometimes have low immunity and are susceptible to certain diseases and are affected by allergens. However, people in rural areas are exposed to harsh environmental conditions therefore develop better immunity.
Question 3.
Why do some adolescents start taking drugs? How can this be avoided?
Answer:
Reasons for drug and alcohol abuse among adolescents are :
(1) Curiosity : Adolescents want to have personal experience of smoking cigarette and taking alcohol and drugs.
(2) Adventure and excitement : A child may go in for use of drug, smoking and alcoholic drink for the sake of adventure and excitement.
(3) Family set up : In certain families, use of alcohol, tobacco, sleeping pills and pain killers are common. It induces the youngsters to taste the
same.
(4) Group or peer pressure : Friends and peer groups often motivate some adolescents to take drugs, alcohol as a defiance of authority and feeling of independence.
(5) Progressiveness : There is a false perception that taking of drugs, alcohol or smoking is a sign of progressiveness in society.
Various measures which help to avoid drug abuse are:
(1) Discipline : Good nurturance with consistent discipline but without suffocating strictness reduces the risk of addictions.
(2) Communication : The child must be able to communicate with the parents seeking clarification of all doubts and discussing problems that arise in studies or develop in the class, with friends, siblings and others.
(3) Independent working : Give responsibility to the child for small tasks and allow him/ her to perform independently. Of course, provide guidance where required.
(4) Education and counselling : Stresses, failures, disappointments and problems are part of life. A child has to be trained, educated and counselled to face them as and when they come.
(5) Looking for danger signs : Teachers and parents should always be careful to look for and identify danger signs that can indicate tendency to go in for addiction.
Question 4.
In your locality, if a person is addicted to alcohol, what kind of behavioural changes do you observe in that person? Suggest measures to overcome the problem.
Answer:
Behavioural changes which can be observed in an alcoholic are as follows:
(1) Reckless behaviour, vandalism and violence.
(2) Drop in academic performance and unexplained absence from school/ college.
(3) Lack of interest in personal hygiene, isolation, depression, fatigue, aggressive-ness.
(4) Change in eating and sleeping habits. Measures to overcome the problem of alcohol addiction are as follows:
- Seeking professional advice : Highly qualified psychologists, psychiatrists and de-addiction and rehabilitation programmes can help individuals who are suffering from alcohol abuse.
- Avoid undue peer pressure : Every person has his/her own choice and personality, which should be kept in mind. So he/ she should not be pressed unduly to do beyond his/her capacities, in work condition and other in social get together or activities.
- Education and counselling : Helps to overcome the problems, like stresses, disappointments and failure in life. One should utilise one’s energy in some beneficial activities like sports, music, reading, yoga and other extra curricular activities.
- Seeking help from parents and peers : In case of minors, whenever, there is any problem, one should seek help and guidance from parents and peers. Help should be taken from close and trusted friends. This would help young to share their feelings of anxiety and wrong doings.
Question 5.
What are the methods of cancer detection? Describe the common approaches for treatment of cancer. Answer:
Detection and diagnosis of cancer depends upon histological features of malignant structure. Few methods of cancer detection are as follows:
(1) Bone marrow biopsy and abnormal count of WBCs in leukemia.
(2) Biopsy of tissue, direct or through endoscopy. Pap test (cytological staining) is used for detecting cancer of cervix and other parts of genital tract.
(3) Techniques such as radiography (use of X-rays), CT Scan (computed tomography), MRI Scan (magnetic resonance imaging)
are very useful to detect cancers of the internal organs. Mammography is radiographic examination of breasts for possible cancer.
(4) Monoclonal antibodies coupled to appropriate radioisotopes can detect cancer specific antigens and hence cancer.
Treatment of cancer includes following :
(1) Chemotherapy : In chemotherapy a variety of anti-cancer drugs are used that produce more injury to cancer cells than to normal cells. These drugs interfere with the cell division and growth and affect both normal and cancerous cells. Vincristine and vinblastine from Catharanthus roseus (Vinca rosea) are effective in leukaemia control. Taxol is another anti-cancer drug obtained from Taxus baccata. Tetrathiomolybdate is the new anti-cancer drug. It arrests the tumour growth by starving cancer cells of copper.
(2) Radiotherapy : It is used in addition to chemotherapy. The basic priniciple there is to bombard cancer cells with rays that damage or destroy the ability of cancer cell to grow and divide by damaging the DNA within the tumour cells, but produce minimum damage to the surrounding normal tissue.
(3) Surgery : It is removal of the cancerous cells surgically and has only limited usefulness. In breast tumour and uterine tumour, it is most effective but other treatments are also given to kill any cells that may have been left.
(4) Immunotherapy : Immunotherapy is a form of treatment that enhances the body’s ability to recognise cancer cells and destroy them. It can be given intravenously or by subcutaneous injection.
(5) Blood and marrow transplant : High dose chemotherapy or radiation therapy can destroy bone marrow’s ability to make blood cells. A blood or marrow transplant can be used to replace marrow stem cells which produce blood cells.
Question 6.
Drugs like LSD, barbiturates, amphetamines, etc., are used as medicines to help patients with mental illness. However, excessive doses and abusive usage are harmful. Enumerate the major adverse effects of such drugs in humans.
Answer:
LSD is a psychedelic drug or hallucinogen which induces behavioural abnormalities, they cause optical or auditory hallucinations, horrible dreams, emotional outburst and severe damage to central nervous system.
Barbiturates are sedatives which depress CNS activity, give feeling of calmness, relaxation and drowsiness but high doses induces deep sleep.
Amphetamines are synthetic drugs and are CNS stimulants. They cause alertness, self-confidence, talkativeness, wakefulness and increased work capacity. High doses cause euphoria, marked excitement and sleeplessness which may lead to mental confusion. Their use may produce after effects like nausea and vomiting. Amphetamine is one of the drugs included in the ‘dope test’ for athletes.
Question 7.
What is Pulse Polio Programme of Government of India? What is OPV? Why is it that India is yet to eradicate Polio?
Answer:
Pulse polio programme is a programme that involves vaccination of children in the age group of 0-5 years with polio vaccine. It began in 1995 with the aim to make India a polio-free country. Oral Polio Vaccine (OPV) is a live-attenuated vaccine, produced by the passage of the virus through non-human cells at a sub-physiological temperature, which produces spontaneous mutations in the viral genome. OPV also proved to be superior in administration, eliminating the need for sterile syringes and making the vaccine more suitable for mass vaccination campaigns. OPV also provides long lasting immunity than the Salk vaccine. One dose of OPV produces immunity to all three poliovirus serotypes in approximately 50% of recipients. WHO declared India and the entire South-East Asia region polio free in March, 2014. Last case of polio in India was reported in January, 2011.
Question 8.
What are recombinant DNA vaccines? Give two examples of such vaccines. Discuss their advantages.
Answer:
Recombinant vaccines are vaccines having a gene encoding the protein of pathogen that causes immunogenic reactions in the host so as to produce antibody against the disease. These vaccines are produced from yeast through recombinant DNA technology. Examples are Hepatitis B vaccine and herpes virus vaccine.
Advantages – By rDNA technology the vaccines can be produced on larger scale so providing greater availabilitv for immunisation. These vaccines are highly specific, pure and elicit strong immune response.
We hope the NCERT Exemplar Solutions for Class 12 Biology chapter 8 Human Health and Diseases help you. If you have any query regarding.NCERT Exemplar Solutions for Class 12 Biology chapter 8 Human Health and Diseases, drop a comment below and we will get back to you at the earliest.