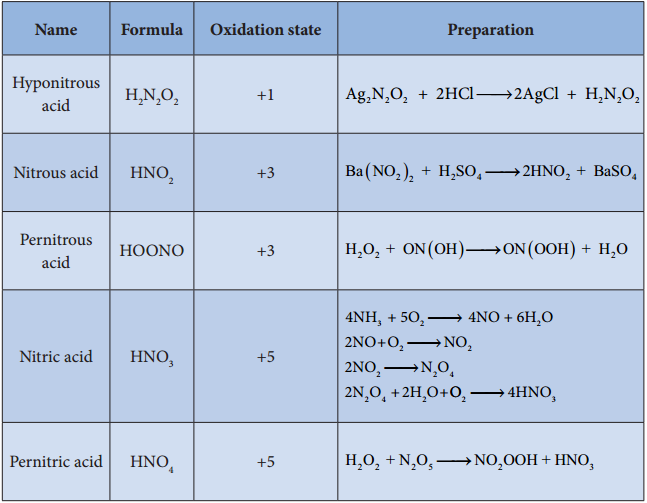
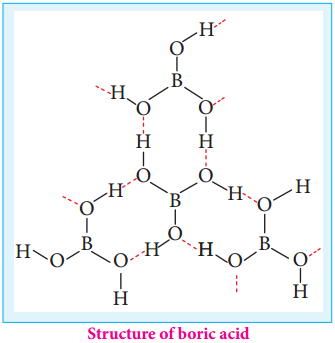
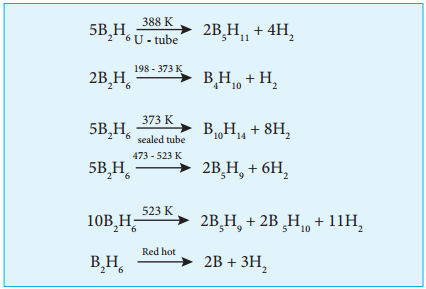
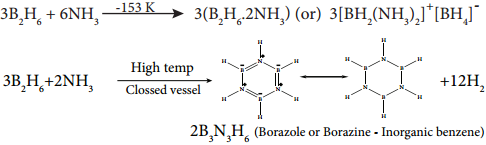
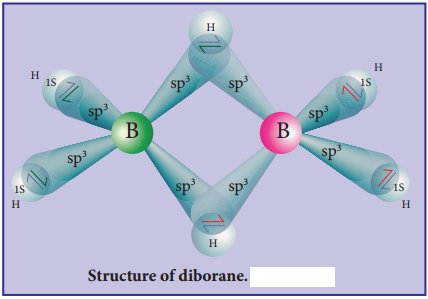
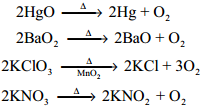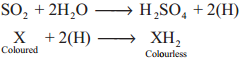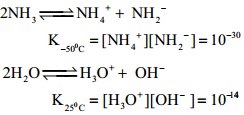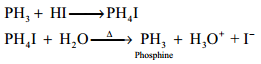Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
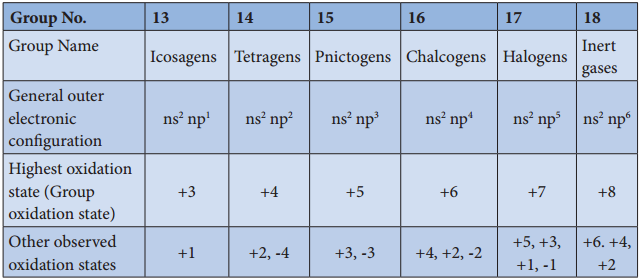
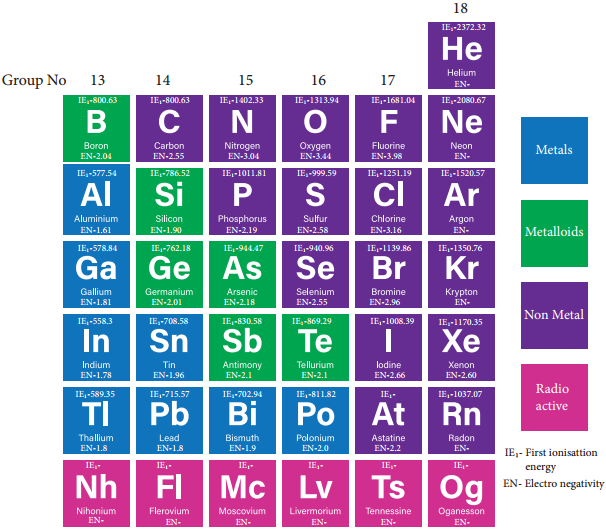
Group 17 (Halogen Group) Elements
Chlorine
Occurrence:
The halogens are present in combined form as they are highly reactive. The main source of flourine is fluorspar or fluorite. The other ores of flourine are cryolite, fluroapatite. The main source of chlorine is sodium chloride from sea water. Bromides and iodides also occur in sea water.
Physical Properties:
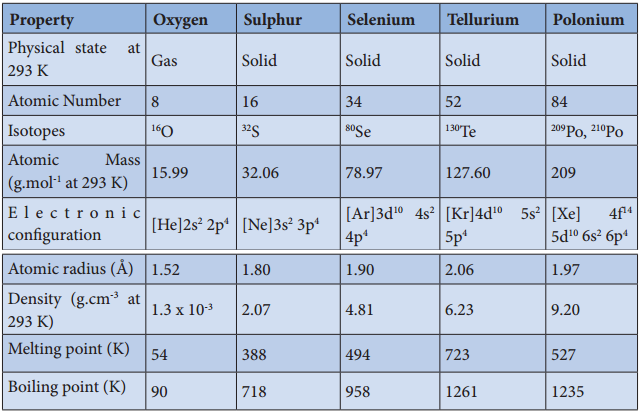
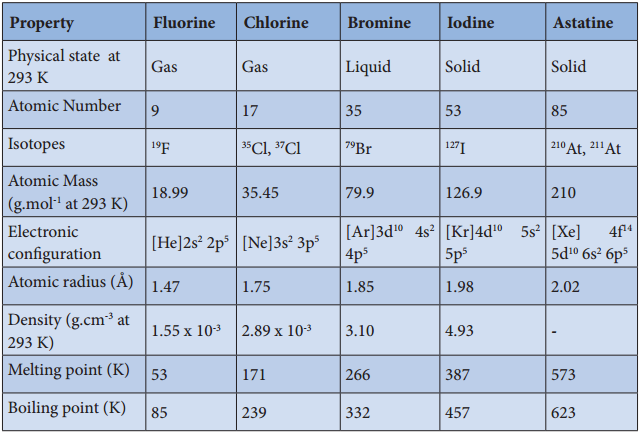
The common physical properties of the group 17 elements are listed in the table.
Physical Properties of Group 17 Elements
Properties:
Chlorine is highly reactive hence it doesn’t occur free in nature. It is usually distributed as various metal chlorides. The most important chloride is sodium chloride which occurs in sea water.
Preparation:
Chlorine is prepared by the action of conc. sulphuric acid on chlorides in presence of manganese dioxide.
4NaCl + MnO2 + 4H2SO4 → Cl2 + MnCl2 + 4NaHSO4 + 2H2O
It can also be prepared by oxidising hydrochloric acid using various oxidising agents such as manganese dioxide, lead dioxide, potassium permanganate or dichromate.
PbO2 + 4HCl → PbCl2 + 2H2O + Cl2
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
When bleaching powder is treated with mineral acids chlorine is liberated
CaOCl2 + 2HCl → CaCl2 + H2O + Cl3
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
Manufacture of Chlorine:
Chlorine is manufactured by the electrolysis of brine in electrolytic process or by oxidation of HCl by air in Deacon’s process.
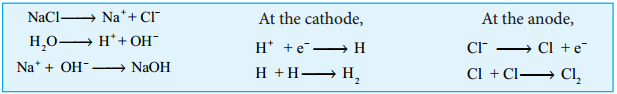
Electrolytic Process:
When a solution of brine (NaCl) is electrolysed, Na+ and Cl– ions are formed. Na+ ion reacts with
OH– ions of water and forms sodium hydroxide. Hydrogen and chlorine are liberated as gases.
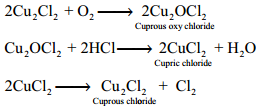
Deacon’s Process:
In this process a mixture of air and hydrochloric acid is passed up a chamber containing a number of shelves, pumice stones soaked in cuprous chloride are placed. Hot gases at about 723 K are passed through a jacket that surrounds the chamber.
![]()
The chlorine obtained by this method is dilute and is employed for the manufacture of bleaching powder. The catalysed reaction is given below,
Physical Properties:
Chlorine is a greenish yellow gas with a pungent irritating odour. It produces headache when inhaled even in small quantities whereas inhalation of large quantities could be fatal. It is 2.5 times heavier than air. Chlorine is soluble in water and its solution is referred as chlorine water. It deposits greenish yellow crystals of chlorine hydrate (Cl2.8H2O). It can be converted into liquid (Boiling point – 34.6°C) and yellow crystalline solid (Melting point – 102°C).
Chemical Properties:
Action with metals and non-metals:
It reacts with metals and non metals to give the corresponding chlorides.
2Na + Cl2 → 2NaCl
2Fe + 3Cl2 → 2FeCl3
2Al + 3Cl2 → 2AlCl3
Cu + Cl2 → CuCl2
H2 + Cl2 → 2HCl; ∆H = – 44kCal
2B + 3Cl2 → 2BCl3
![]()
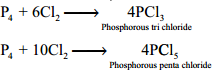
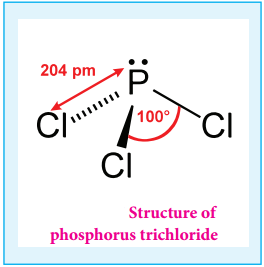
P4 + 6Cl2 → 4PCl3
2As + 3Cl2 → 2AsCl3
2Sb + 3Cl2 → 2SbCl3
Affinity for Hydrogen:
When burnt with turpentine it forms carbon and hydrochloric acid.
C10H16 + 8Cl2 → 10C + 16HCl
It forms dioxygen when reacting with water in presence of sunlight. When chlorine in water is exposed to sunlight it loses its colour and smell as the chlorine is converted into hydrochloric acid.
2Cl2 + 2H2O → O2 + 4HCl
Chlorine reacts with ammonia to give ammonium chloride and other products as shown below:
With excess ammonia,
2NH3 + 3Cl2 → N2 + 6HCl
6HCl + 6H3 → 6NH4Cl
Overall Reaction
8NH3 + 3Cl2 → N2 + 6NH4Cl
With excess chlorine,
NH3 + 3Cl2 → NCl3 + 3HCl
3HCl + 3NH3 → 3NH4Cl
Overall Reaction
4NH3 + 3Cl2 → NCl3 + 3HCl
3HCl + 3HCl3 → 3NH4Cl
Chlorine oxidises hydrogen sulphide to sulphur and liberates bromine and iodine from iodides and bromides. However, it doesn’t oxidise fluorides.
H2S + Cl2 → 2HCl + S
Cl2 + 2KBr → 2KCl + Br2
Cl2 + 2KI → 2KCl + I2
Reaction with Alkali:
Chlorine reacts with cold dilute alkali to give chloride and hypochlorite while with hot concentrated alkali chlorides and chlorates are formed.
Cl2 + H2O → HCl + HOCl
HCl + NaOH → NaCl + H2O
HOCl + NaOH → NaOCl + H2O

(Cl2 + H2O → HCl + HOCl) × 3
(HCl + NaOH → NaCl + H2O) × 3
(HOCl + NaOH → NaOCl + H2O) × 3
3NaOCl → NaClO3 + 2NaCl

Oxidising and Bleaching Action:
Chlorine is a strong oxidising and bleaching agent because of the nascent oxygen.
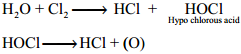
Colouring matter + Nascent oxygen → Colourless oxidation product
The bleaching of chlorine is permanent. It oxidises ferrous salts to ferric, sulphites to sulphates and hydrogen sulphide to sulphur.
2FeCl2 + Cl2 → 2FeCl3
Cl2 + H2O → HCl + HOCl
2FeSO4 + H2SO4 + HOCl → Fe2(SO4)3 + HCl + H2O
![]()
Cl2 + H2O → HCl + HOCl
Na2SO3 + HOCl → Na2SO4 + HCl
Overall Reaction
Na2SO3 + H2O + Cl2 → CaOCl2 + H2O
Preparation of Bleaching Powder:
Bleaching powder is produced by passing chlorine gas through dry slaked lime (calcium hydroxide).
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Displacement Redox Reactions:
Chlorine displaces bromine from bromides and iodine from iodide salts.
Cl2 + 2KBr → 2KCl + Br2
Cl2 + 2KI → 2KCl + I2
Formation of Addition Compounds:
Chlorine forms addition products with sulphur dioxide, carbon monoixde and ethylene. It forms substituted products with alkanes/arenes.
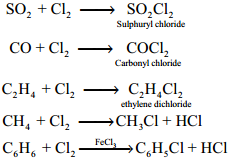
Uses of Chlorine:
It is used in
- Purification of drinking water
- Bleaching of cotton textiles, paper and rayon
- Extraction of gold and platinum
Hydrochloric Acid:
Laboratory Preparation:
It is prepared by the action of sodium chloride and concentrated sulphuric acid.
NaCl + H2SO4 → NaHSO4 + HCl
NaHSO4 + NaCl → Na2SO4 + HCl
Dry hydrochloric acid is obtained by passing the gas through conc. sulphuric acid.
Properties:
Hydrogen chloride is a colourless, pungent smelling gas, easily liquefied to a colourless liquid (boiling point 189K) and frozen into a white crystalline solid (melting point 159K). It is extremely soluble in water.
HCl (g) + H2O (l) → H3O+ + Cl–
Chemical Properties:
Like all acids it liberates hydrogen gas from metals and carbon dioxide from carbonate and bicarbonate salts.
Zn + 2HCl → ZnCl2 + H2
Mg + 2HCl → MgCl2 + H2
Na2CO3 + 2HCl → 2NaCl + CO2 + H2
CaCO3 + 2HCl → CaCl2 + CO2 + H2
NaHCO3 + 2HCl → 2NaCl + CO2 + H2O
It liberates sulphur dioxide from sodium sulphite
Na2SO3 + 2HCl → 2NaCl + H2O + SO2
When three parts of concentrated hydrochloric acid and one part of concentrated nitric acid are mixed, Aquaregia (Royal water) is obtained. This is used for dissolving gold, platinum etc.
Au + 4H+ + NO3– + 4Cl– → AuCl–4 + NO + 2H2O
3Pt + 16H+ + 4NO3– + 18Cl– → 3[PtCl6]2- + 4NO + 8H2O
Uses of Hydrochloric Acid:
- Hydrochloric acid is used for the manufacture of chlorine, ammonium chloride, glucose from corn starch etc.,
- It is used in the extraction of glue from bone and also for purification of bone black
Trends in Physical and Chemical Properties of Hydrogen Halides:
Preparation:
Direct combination is a useful means of preparing hydrogen chloride. The reaction between hydrogen and flourine is violent while the reaction between hydrogen and bromine or hydrogen and iodine are reversible and don’t produce pure forms.
Displacement Reactions:
Concentrated sulphuric acid displaces hydrogen chloride from ionic chlorides. At higher temperatures the hydrogen sulphate formed react with further ionic chloride. Displacement can be used for the preparation of hydrogen flourides from ionic flourides. Hydrogen bromide and hydrogen iodide are oxidised by concentrated sulphuric acid and can’t be prepared in this method.
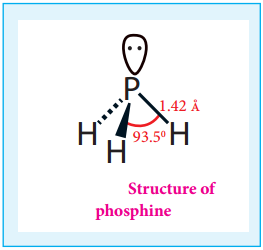
Hydrolysis of Phosphorus Trihalides:
Gaseous hydrogen halides are produced when water is added in drops to phosphorus tri halides except phosphorus triflouride.
PX3 + 3H2O → H3PO3 + 3HX
Hydrogen bromide may be obtained by adding bromine dropwise to a paste of red phosphorous and water while hydrogen iodide is conveniently produced by adding water dropwise to a mixture of red phosphorous and iodine.
2P + 3X2 → 2PX3
2PX3 + 3H2O → H3PO3 + 3HX (where X = Br or I)
Any halogen vapours which escapes with the hydrogen halide is removed by passing the gases through a column of moist red phosphorous.
From Covalent Hydrides:
Halogens are reduced to hydrogen halides by hydrogen sulphide.
H2S + X2 → 2HX + S
Hydrogen chloride is obtained as a by-product of the reactions between hydrocarbon of halogens.
General Properties:
In line with the decreasing bond dissociation enthalpy, the thermal stability of hydrogen halides decreases from flouride to iodide. For example, Hydrogen iodide decomposes at 400° C while hydrogen flouride and hydrogen chloride are stable at this temperature.
At room temperature, hydrogen halides are gases but hydrogen flouride can be readily liquefied. The gases are colourless but, with moist air gives white fumes due to the production of droplets of hydrohalic acid. In HF, due to the presence of strong hydrogen bond it has high melting and boiling points. This effect is absent in other hydrogen halides.
|
HF |
HCl | HBr |
HI |
|
| Bond dissociation enthalphy (kJ mol-1) | +562 | +431 | +366 | +299 |
| % of ionic character | 43 | 17 | 13 | 7 |
Acidic Properties:
The hydrogen halides are extremely soluble in water due to the ionisation.
HX + H2O → H3O+ + X–
(X – F, Cl, Br, or I)
Solutions of hydrogen halides are therefore acidic and known as hydrohalic acids. Hydrochloric, hydrobromic and hydroiodic acids are almost completely ionised and are therefore strong acids but HF is a weak acid i.e. 0.1mM solution is only 10% ionised, but in 5M and 15M solution HF is stronger acid due to the equilibrium.
HF + H2O ⇄ H3O+ + F–
HF + F– ⇄ HF–2
At high concentration, the equilibrium involves the removal of flouride ions is important. Since it affects the dissociation of hydrogen flouride and increases and hydrogen ion concentration Several stable salts NaHF2, KHF2 and NH4HF2 are known. The other hydrogen halides do not form hydrogen dihalides.
Hydrohalic acid shows typical acidic properties. They form salts with acids, bases and reacts with metals to give hydrogen. Moist hydroflouric acid (not dry) rapidly react with silica and glass.
SiO2 + 4HF → SiF4 + 2H2O
Na2SiO3 + 6HF → Na2SiF6 + 3H2O
Oxidation:
Hydrogen iodide is readily oxidised to iodine hence it is a reducing agent.
2HI ⇄ 2H+ + I2 + 2e–
Acidic solution of iodides is readily oxidised. A positive result is shown by liberation of iodine which gives a blue-black colouration with starch.
Hydrogen bromide is more difficult to oxidise than HI. HBr reduces slowly H2SO4 into SO2
2HBr + H2SO4 → 2H2O + Br2 + SO2
But hydrogen iodide and ionic iodides are rapidly reduced by H2SO4 into H2S and not into SO2.
8HI + H2SO4 → 4H2O + 4I2 + H2S
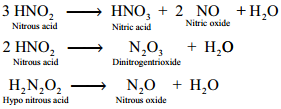
Reducing property of hydrogen iodide can be also explained by using its reaction with alcohols into ethane. It converts nitric acid into nitrous acid and dinitrogen dioxide into ammonium.
Hydrogen chloride is unaffected by concentrated sulphuric acid but affected by only strong oxidising agents like MnO2, potassium permanganate or potassium chloride.
To summarize the trend,
|
Property |
Order |
| Reactivity of hydrogen | Decreases from fluorine to iodine |
| Stability | Decreases from HF to HI |
| Volatility of the hydrides | HF < HI < HBr < HCl |
| Thermal Stability | HF > HI > HBr > HCl |
| Boiling Point | HCl < HBr < HI |
| Acid strength | Increases from HF to HI |
Inter Halogen Compounds:
Each halogen combines with other halogens to form a series of compounds called inter halogen compounds. In the given table of inter halogen compounds a given compound A is less electronegative than B.
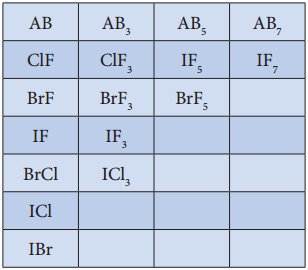
Properties of Inter Halogen Compounds:
- The central atom will be the larger one
- It can be formed only between two halogen and not more than two halogens
- Fluorine can’t act as a central metal atom being the smallest one
- Due to high electronegativity with small size florine helps the central atom to attain high coordination number
- They can undergo the auto ionization.
- They are strong oxidising agents
2 ICI ⇄ I+ + ICI–2
2 ICI ⇄ ICI+2 + ICI4–
Reaction with Alkali:
When heated with the alkalis, larger halogen form oxyhalogens and the smaller forms halide.
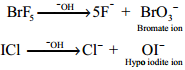
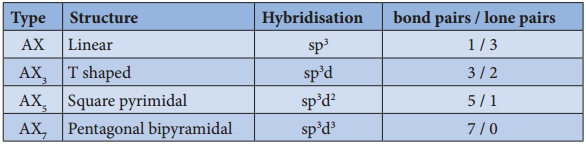
Structure of Inter Halogen Compounds:
The structures of different type of interhalogen compunds can be easily explained using VSEPR theory. The details are given below.
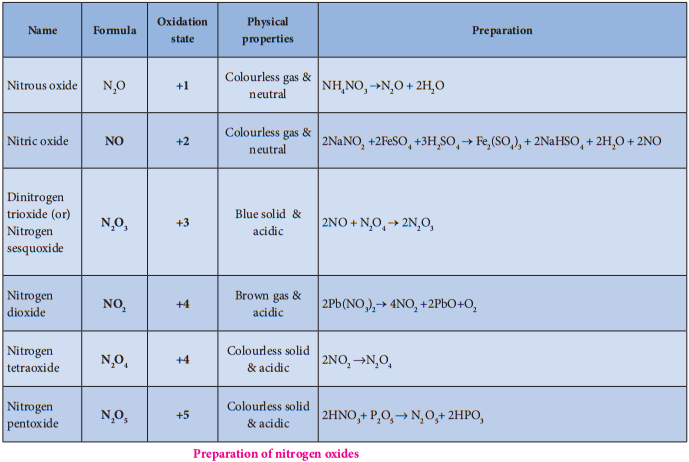
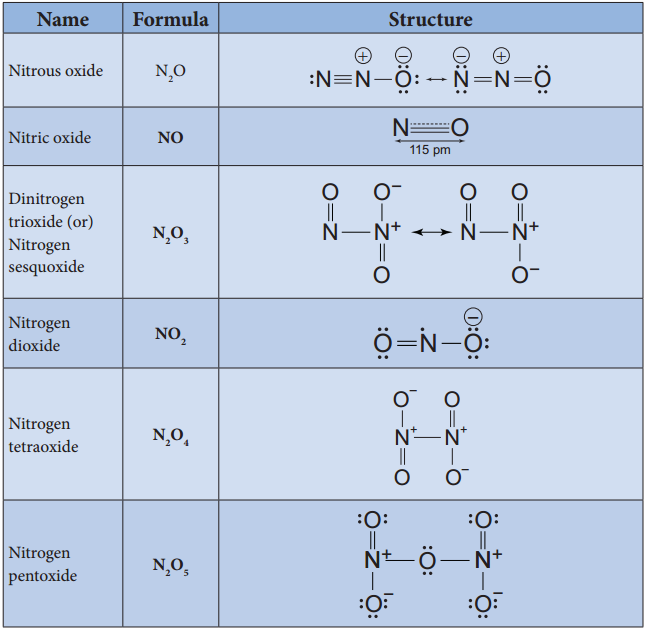
Oxides of Halogen
Fluorine reacts readily with oxygen and forms difluorine oxide (F2O) and diflourine dioxide (F2O2) where
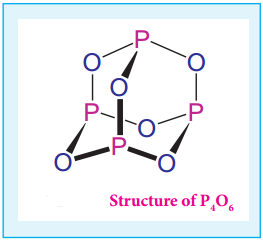
it has a – 1 oxidation state. Other halogens do not react with oxygen readily. But the following oxides can be prepared by some indirect methods. Except flourine all the other halogens have positive oxidation states.
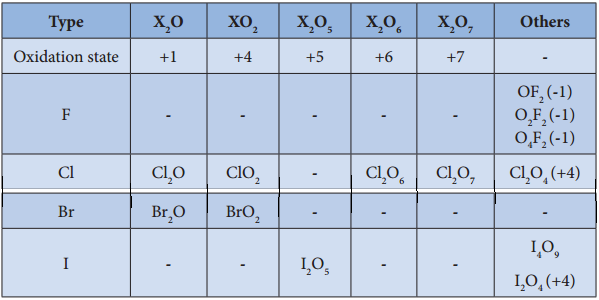
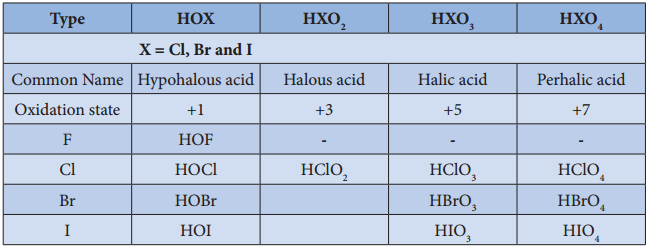
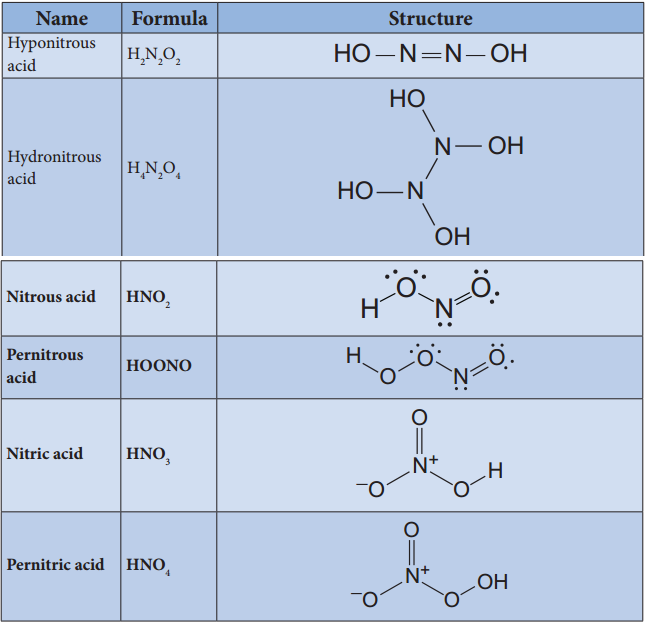
Oxoacids of Halogens:
Chlorine forms four types of oxoacids namely hypochlorus acid, chlorous acid, chloric acid and perchloric acid. Bromine and iodine forms the similar acids except halous acid. However, flourine only forms hypofulric acid. The oxidizing power oxo acids follows the order:
HOX > HXO3 > HXO3 > HXO4