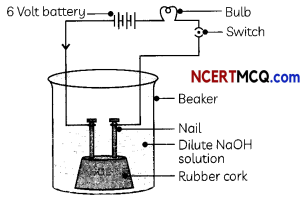
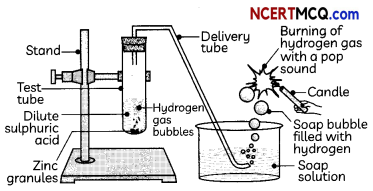
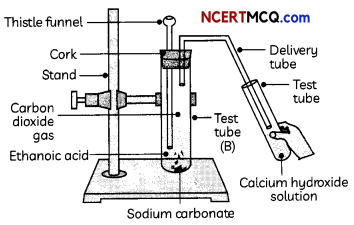
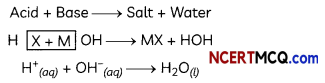Indicators
These are substances that tell us whether a substance is an acid or a base by change in colour or odour when added into the substance.
Types of Indicators:
Indicators are classified in the following ways:
Natural Indicators
Natural indicators are the substances obtained from natural sources such as a dye, flower, leaf, etc., in plants.
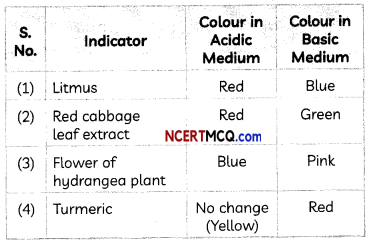
Examples: Litmus (a purple coloured dye extracted from the lichen plant), red cabbage leaf extract, etc.
The table below shows the effect of some common natural indicators on acids and bases:
Important
The yellow stain of curry on a white cloth, which turns reddish-brown when scrubbed with soap is due to the fact that soap solution changes the colour of turmeric to red- brown as it is basic in nature. And when the cloth is rinsed with water, the basic soap gets removed and the stain turns to yellow again.
![]()
Example 1.
You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Answer:
Label the three test tubes as A, B and C. Take a small amount of each of the three given liquids in these test tubes A, B and C. First, dip red litmus paper one by one in each test tube. The liquid which will turn red litmus paper blue is basic in nature.
Remove this test tube as it has been identified as containing a basic solution.
Next, put a drop of solution from the remaining two test tubes on the blue litmus paper (which was earlier red in colour but turned blue on adding basic solution to it).
The solution which turns blue litmus paper red is acidic in nature. The solution that does not change the colour of either red litmus or blue litmus is neutral in nature, which is distilled water.
Synthetic Indicators:
Synthetic indicators are the compounds synthesized in a chemistry lab or industrially rather than compounds found in nature.
Examples: Phenolphthalein, Methyl orange.
The table below shows the effect of some common synthetic indicators on acids and bases:

![]()
Olfactory Indicators:
These are the substances whose odour or smell changes in acidic or basic medium.
Examples: Onion extract, vanilla extract. The table beLow shows the effect of some common olfactory indicators on acids and bases:


Important
Olfactory indicators ore used to ensure the participation of visually impaired students ¡n the Laboratory for distinguishing between acidic and basic substances