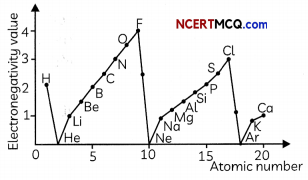
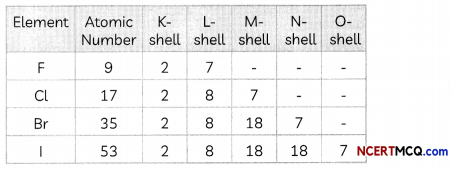
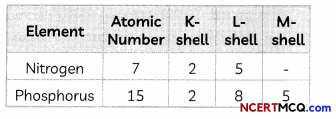
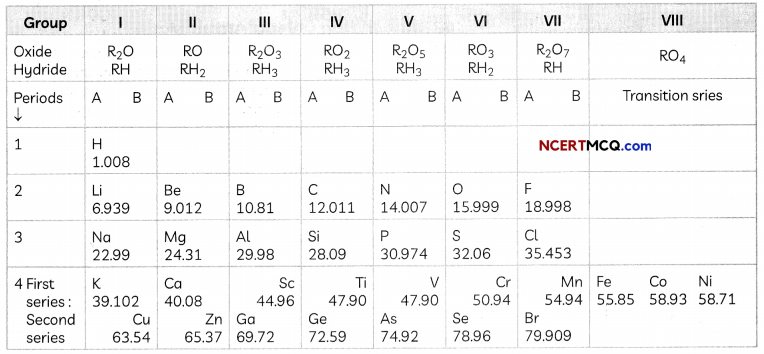
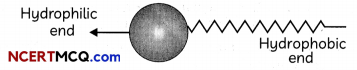
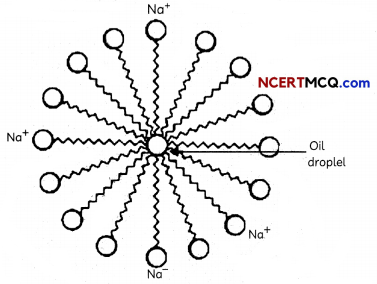
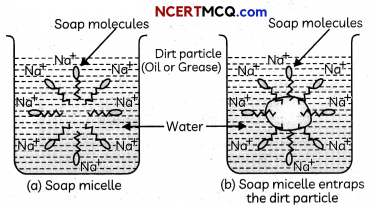
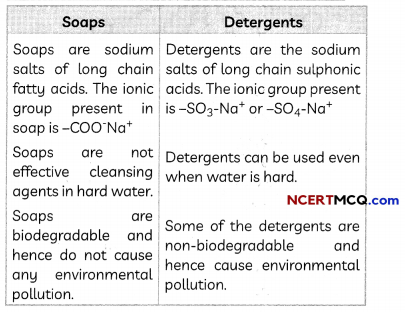
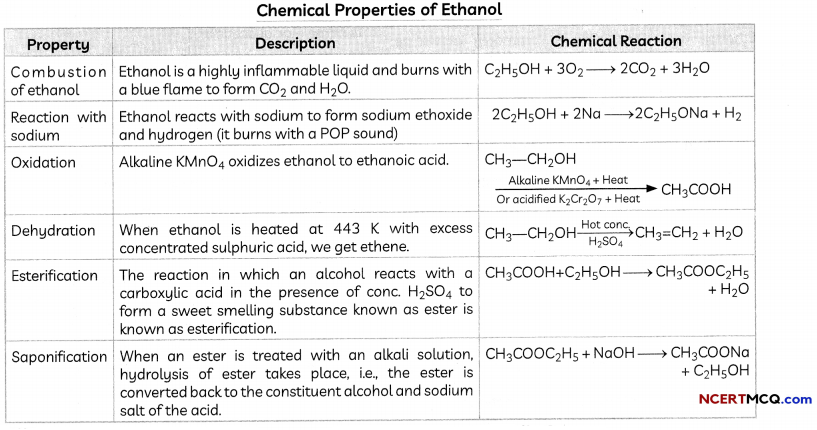
Transportation
Transportation in Human Beings
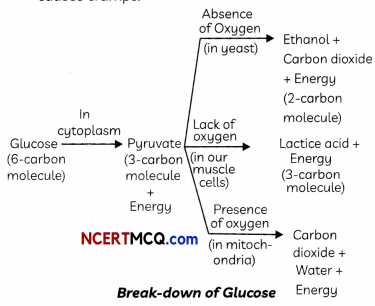
In human beings, the transportation of various substances to relevant organs and tissues is done by blood and lymph.
- Blood circulatory system: The blood circulatory system comprises heart and blood vessels.
- Blood: Blood is a connective tissue having a fluid matrix called plasma and three kinds of cells-Red Blood cells, White Blood cells and Blood platelets.
Functions of Blood
- Blood is responsible for the transportation of nutrients, respiratory gases, waste products, hormones, enzymes and ions from one part of the body to the other.
- Blood plays a role in temperature regulation and protection of the body from the attack of foreign bodies and disease-causing pathogens.
- Blood forms a clot at the site of injury thus preventing further loss of blood.
| Component of Blood | Function |
| Plasma | Plasma is colourless. It contains a lot of water and many proteins. The blood cells (WBC, RBC & Platelets) float in this fluid matrix. Plasma without fibrinogen is called serum. |
| Red Blood Corpuscles | Also known as Erythrocytes, these contain the red coloured pigment haemoglobin, due or RBCto which the blood looks red. These are produced in the bone marrow. |
| White Blood Corpuscles or WBC | Also known as Leucocytes, these are lesser in number than RBC. These cells protect the body from infections. |
| Platelets | These also manufacture antibodies, which are responsible for immunity. These are fragments of cells and do not possess nuclei. They participate in the coagulation of blood. |
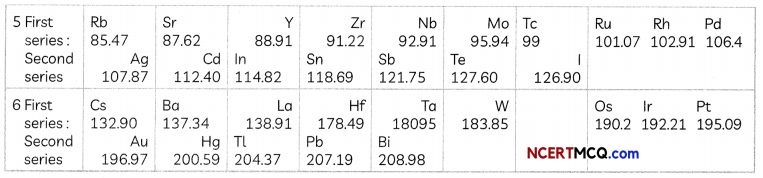
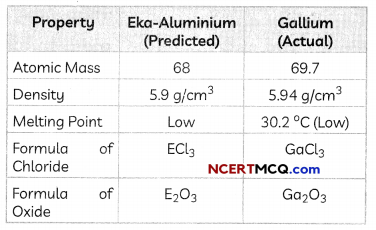
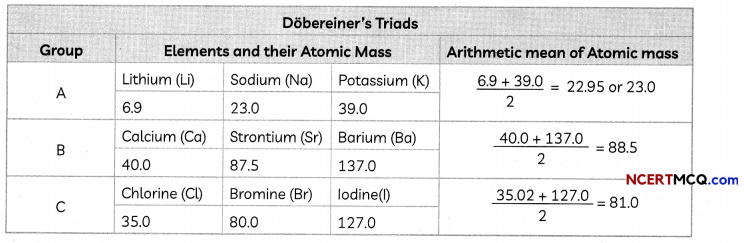
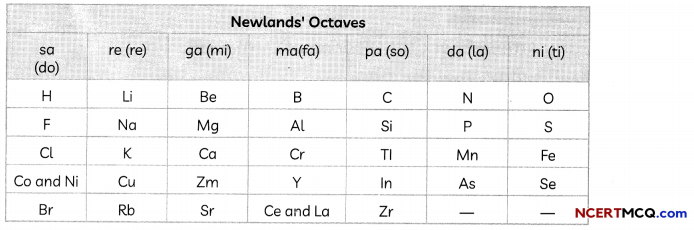
The Human Heart
The heart is a muscular organ which is as big as our fist. Because both oxygen and carbon dioxide have to be transported by the blood, the heart has different chambers to prevent the oxygen-rich blood from mixing with the blood containing carbon dioxide.
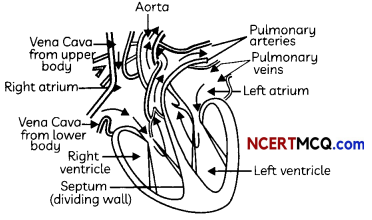
A schematic sectional view of the human heart
Structure of Heart: The Human Heart consists of four chambers:
- Two Upper Chambers: Two upper chambers are called atria or auricles. These are right atrium and left atrium. These receive blood from large veins. Vene cava opens in right auricle (RA) and pulmonary veins open in left auricle (LA).
- Two Lower Chambers: Two lower chambers are called ventricles- left ventricle (LV) and right ventricle (RV). These transport blood to lungs and the entire body. These chambers are separated by partitions called septa.
![]()
Double circulation:
The working of the heart is described below:
- The cardiac muscles of all the four chambers of heart relax.
- Deoxygenated Blood from large veins, called vena cava, pour into RA.
- Pulmonary veins from lungs pour oxygenated blood into LA.
- The atria contract.
- RA pours deoxygenated blood into RV and LA pours oxygenated blood into LV.
- The ventricles contract.
- Oxygenated blood from LV is distributed to all parts of the body through aorta.
- Deoxygenated blood flows to the lungs from RV through the pulmonary artery.
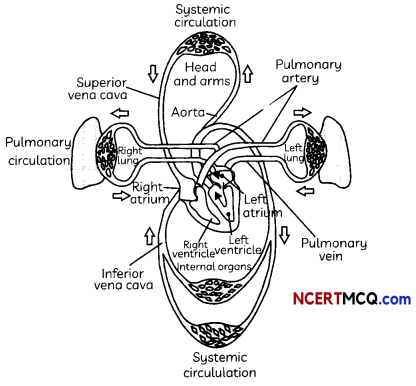
We see that the deoxy noted blood comes to the heart, it is oxygenated in the lungs and comes back to the heart from where it is distributed to all parts of the body. This flow of blood twice through the heart is known as double circulation.
Ventricles have thicker muscular walls: Since ventricles have to pump blood into various organs, they have thicker muscular walls than the atria do. Valves ensure that blood does not flow backwards when the atria or ventricles contract.
Transport and exchange of oxygen and carbon dioxide: The separation of the right side and the left side of the heart is useful to keep oxygenated and deoxygenated blood from mixing as such separation allows a highly efficient supply of oxygen to the body. This is useful in animals that have high energy needs, such as birds and mammals, which constantly use energy to maintain their body temperature.
Animals like amphibians or many reptiles have three-chambered hearts and tolerate some mixing of the oxygenated and de-oxygenated blood streams.
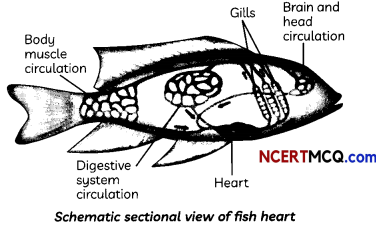
Structure of Heart in Fish: Fishes have only two chambers to their hearts, and the blood is pumped to the gills, is oxygenated there, and passes directly to the rest of the body. Thus, blood goes only once through the heart in the fish during one cycle of passage through the body.
Blood pressure: The force that blood exerts against the wall of a vessel is called blood pressure. This pressure is much greater in arteries than in veins. The pressure of blood inside the artery during ventricular systole (contraction) is called systolic pressure and pressure in artery during ventricular diastole (relaxation) is called diastolic pressure. The normal systolic pressure is about 120 mm of Hg and diastolic pressure is 80 mm of Hg.
The normal blood pressure values are:
- Systolic pressure: 120 mm Hg
- Diastolic Pressure: 80 mm Hg
- This is usually written as 120/80 mm Hg.
- Blood pressure is measured with an instrument called a sphygmomanometer.
Hypertension: High blood pressure is also called hypertension and is caused by the constriction of arterioles, which results in increased resistance to blood flow. It can lead to the rupture of an artery and internal bleeding.
![]()
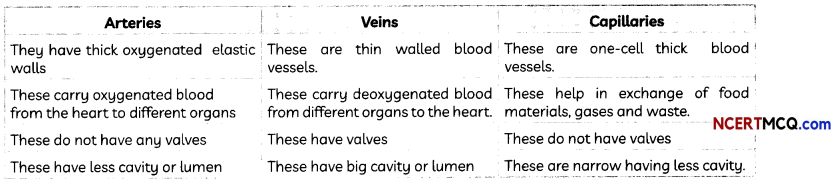
Blood vessels: Blood vessels are of three kinds:
- Arteries
- Veins
- Capillaries
A brief summary of their features is given below:
Maintenance by platelets: These are cells present in the blood which circulate around the body and help the blood to clot at the points of injury.
Lymphatic system: Lymphatic system is regarded as second circulatory system of the human body and consists of the following:
- Lymphatic glands and lymph nodes
- Lymph vessels and capillaries
- Lymph
Lymph: Lymph or tissue fluid is a yellow coloured circulatory fluid that flows in the lymphatic capillaries, which join to form large lymph vessels. It is formed when some amount of plasma, proteins and blood cells escape into intercellular spaces in the tissues through the pores present in the walls of capillaries.
It is similar to the plasma of blood but colourless and contains less protein. Lymph drains into lymphatic capillaries from the intercellular spaces, which join to form large lymph vessels that finally open into larger veins.
Functions of lymph
- It contains lymphocyte cells that fight against infections.
- It flows only in one direction-from tissue to the heart.
- It is called extracellular fluid ac it lies outside cells and bathes the cells.
- It returns proteins and plasma from circulation to fluids.
- It carries digested fat.
- It drains excess fluid from the extracellular space back into the blood.
Differences between blood and lymph:
| Blood | Lymph |
| 1. It is a fluid connective | 1. It is an extracellular fluid tissue |
| 2. It consists of plasma and blood Cells | 2. It consists of matrix and lymphocytes |
| 3. It is red in colour | 3. It is colourless |
| 4. It flows in both directions, i.e., from tissues to heart and back | 4. It flows only in one direction, i.e., from tissue to heart |
Electrocardiograph: It is an instrument which can record the electrical changes during a heartbeat. The muscle fibres of the heart generate electric currents due to which the heart beats rhythmically. The graphic recording is called ECG or Electrocardiogram.
Pacemaker: It is a machine that is inserted in a heart patient whose heart does not beat normally. It actually takes the place of the specialized muscle cells that initiate heartbeat in the patient in which they have stopped functioning.
![]()
Transportation in Plants
Need for Proper System of Transportation in Plants
The raw materials absorbed by a plant such as nitrogen, phosphorus and other minerals have to be transported to different plant parts as diffusion processes will not be sufficient to provide raw material in leaves and energy in roots. A proper system of transportation is therefore essential in such situations.
Energy needs Differ between Different Body Designs Plants do not move, and plant bodies have a large proportion of dead cells in many tissues. As a result, plants have low energy needs and can use relatively slow transport systems. The distances over which transport systems have to operate, however, can be very large in plants such as very tall trees.
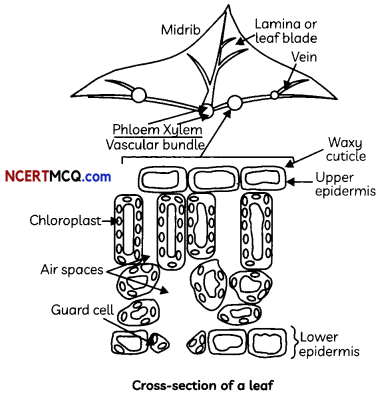
Transport of Water
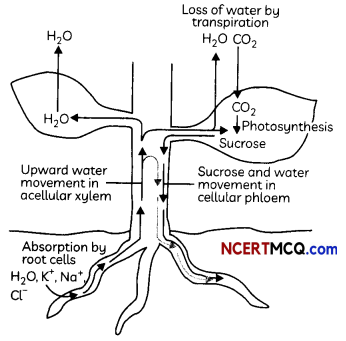
The xylem moves water and minerals obtained from the soil. Water and minerals are transported from the soil to the various parts of the plant by tracheids and vessels – the two elements of xylem, which are both non-living conducting tissues.
Mechanism of Transport in Xylem: In xylem tissue, vessels and tracheids of the roots, stems and leaves are interconnected to form a continuous system of water-conducting channels reaching all parts of the plant.
At the roots, cells in contact with the soil actively take up ions.
This creates a difference in the concentration of these ions between the root and the soil.
Water, therefore, moves into the root from the soil to eliminate this difference.
Thus, there is thus a steady movement of water into root xylem, creating a column of water that is steadily pushed upwards.
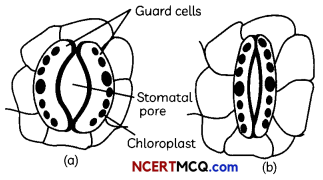
Transpiration
The loss of water in the form of vapour from the aerial parts of the plant is known as transpiration.
The water which is lost through the stomata is replaced by water from the xylem vessels in the leaf Evaporation of water molecules from the cells of a leaf creates a suction that pulls water from the xylem cells of roots.
Importance of transpiration:
1. Transpiration helps in the absorption and upward movement of water and minerals dissolved in it from roots to the leaves.
2. It also helps in temperature regulation. The effect of root pressure in the transport of water is more important at night. During the day when the stomata are open, the transpiration pull becomes the major driving force in the movement of water in the xylem.
Transport of Food and other Substances
The tronsport of soLubLe products of photosynthesis is catted translocation and it occurs in phLoem. The phloem transports products of photosynthesis from the leaves where they are synthesized to other parts of the plant. Besides the products of photosynthesis. the phloem transports amino acids and other substances.
These substances ore especiotty delivered to the storage organs of roots, fruits and seeds and to growing organs.
The translocation of food and other substances takes place in the sieve tubes with the help of adjacent companion cells both in upward and downward directions.
Mechanism of Translocation: The translocation in phloem is achieved by utiLising energy.
- Material Like sucrose is transferred into phloem tissue using energy Prom ATP.
- This increases the osmotic pressure of the tissue causing water to move into it. This pressure moves the material in the phloem to tissues which have Less pressure.
This allows the phloem to move material according to the plants needs.
For example, in the spring, sugar stored in root or stern tissue would be transported to the buds which need energy to grow.
![]()
Example 1.
What are the differences between the transport of materials in xylem and phloem?
Answer:
The differences between the transport of materials in xylem and phloem are:
| Xylem | Phloem |
| 1. Xylem helps in the transportation of water and dissolved minerals from roots to leaves and other plant parts. | 1. Phloem helps in the transportation of dissolved products of photosynthesis from Leaves to other ports of the plant. |
| 2. In xylem, the transport of material takes place through vessels and tracheids which are dead tissues. | 2. In phloem, transport of material takes place through sieve tubes with the help of companion cells, which are living cells. |
| 3. In the xylem, upward movement of water and dissolved minerals is mainly achieved due to Osmotic pressure at roots along with transpiration in which suction is created by evaporation of water from the surface of the Leaf. | 3. In translocation material is transferred into phloem tissue using energy from ATP. This increases the as osmotic pressure that moves the material in the phloem to the tissues which have Less pressure. |
| 4. Movement of water is achieved by simple physical forces. There is no requirement of energy in the form of ATP | 4. The translocation in phloem is an active process and requires energy in the form of ATP |