HOTS Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure
These Solutions are part of HOTS Questions for Class 9 Science. Here we have given HOTS Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure.
Question 1.
A house wife churned full cream with a milk churner
- What will she observe after churning the milk ?
- What could be the possible reason for the observation ?
Answer:
- Churning of milk is a centrifugation process. As a result, lighter particles of cream or butter will move upwards and collect at the top. Heavier residual particles will remain at the bottom. This is a very common process used to separate cream from milk or butter from yogurt.
- The separation is based on the principle that lighter particles move upwards while denser particle downwards upon centrifugation.
More Resources
- HOTS Questions for Class 9 Science
- NCERT Solutions for Class 9 Science
- Value Based Questions in Science for Class 9
- NCERT Exemplar Solutions for Class 9 Science
- Previous Year Question Papers for CBSE Class 9 Science
Question 2.
Based on separation techniques, complete the following. The first one is done for you.
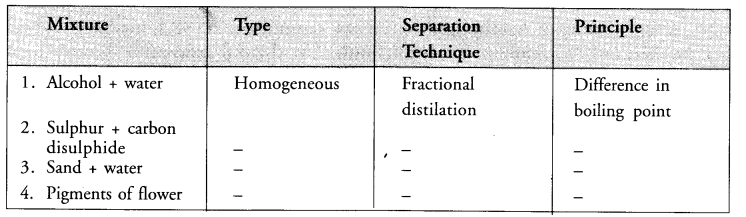
Answer:
2. Homogeneous, Evaporation, Difference in nature.
3. Hetereogeneous, Filtration, Difference in solubility in water
4. Homogeneous, Chromatography, Difference in adsorption of different components.
Question 3.
The table given below shows number of grams of five different solids dissolving in 100 g of the solvents : water, alcohol and chloroform (all at 20°C).
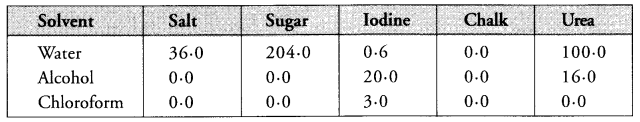
- Which solid dissolves best in water at 20°C ?
- Which solid is maximum soluble in alcohol ?
- Which solid is insoluble in all the three solvents ?
Answer:
- Sugar is best soluble in water at 20°C
- Iodine is maximum soluble in alcohol.
- Chalk is insoluble in all the three solvents.
Question 4.
Some solids dissolve easily in liquids while the others donot
- What is the name given to the liquids which dissolve solids ?
- What is the name given to the clear liquid formed when a solid dissolves in a liquid ?
- What is the name given to the liquid which contains in it some suspended particles ?
Answer:
- The liquids are known as solvents
- The clear liquid is called solution or true solution. .
- The liquid is known as dispersion medium or dispersing medium.
Question 5.
Butter is an example of one type of colloidal solution. Name it. Give a reason for your choice.
Answer:
The colloidal solution is an example in which solid acts as the dispersion medium while liquid as the dispersed phase. It is also called gel.
Reason for the choice. On pressing butter, liquid drops come out of it leaving behind a solid. This clearly shows that butter is a gel.
Question 6.
- The solubility of sodium chloride in water increases with rise in temperature while that of lithium carbonate decreases. Assign reason.
- Water containing 88-8% oxygen and 11-2% hydrogen is often used as a fire extinguisher. Can a mixture containing the two gases in the same ratio by mass be used for extinguishing fire ?
- The melting point of a solid when determined experimentally comes out to be 160°C. But its actual melting point as given in standard books is 150°C. Predict the nature of the solid.
Answer:
- When sodium chloride is dissolved in water, the process is endothermic in nature. This means that heat energy is absorbed in the process. Therefore, solubility increases with rise in the temperature. In case of lithium carbonate, the process of dissolution is exothermic. This means that heat is evolved in the process. Therefore, its solubility in water decreases with rise in temperature.
- No, it cannot be used. Actually, in water the two elements are chemically combined with each other. They therefore, lose their identity. But in the mixture, no chemical combination between hydrogen and oxygen has taken place. Even water cannot be formed on mixing the gases. Therefore, the mixture does not extinguish any fire.
- Since the experimentally determined melting point of the solid is more than the standard value of the melting point, this means that the solid is not in pure state. It has some impurities present. Please note that the purity of a solid can be determined by finding its melting point and comparing it with the standard value.
Hope given HOTS Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.