Chapter 14 Natural Resources Class 9 Science Important Questions with Answers PDF will help you in scoring more marks in your exams.
Natural Resources Class 9 Important Questions and Answers Science Chapter 14
Very Short Answer Questions
Question 1.
Name any two factors responsible for the formation of soil.
(CCE 2011)
Answer:
Abiotic (sun, water, wind) and biotic (living organisms).
More Resources
- Previous Year Question Papers for CBSE Class 9 Science
- NCERT Solutions for Class 9 Science
- NCERT Exemplar Solutions for Class 9 Science
- Value Based Questions in Science for Class 9
- HOTS Questions for Class 9 Science
Question 2.
Write full form of CFC. (CCE 2011)
Answer:
Chloro-fluoro-carbon.
Question 3.
We are lucky that ozone is not stable near to the earth’s surface. Why ? Give appropriate answer. (CCE 2011)
Answer:
Ozone occurring near the surface of the earth is a strong oxidant causing corrosion of articles, hardening of rubber, respiratory distress and internal haemorrhages. Fortunately, it is not stable forming oxygen and oxides.
Question 4.
How is biosphere a dynamic and stable system ?
(CCE 2011)
Answer:
There is a regular circulation of biogeochemicals between the different components of biosphere which also receives regular supply of energy as well that is ultimately dissipated in the form of infra-red rays.
Question 5.
Name the carbon compounds responsible for causing ozone hole in the atmosphere. (CCE 2011, 2012)
Answer:
Chlorofluorocarbons (CFCs).
Question 6.
Name the bacteria responsible for nitrification in nature.
(CCE 2011)
Answer:
Nitrosomonas (ammonia to nitrite) and Nitrobacter (oxidises nitrite to nitrate).
Question 7.
Which gas is the major component of atmosphere of Vends and Mars ? (CCE 2011)
Answer:
Carbon dioxide, 95-97%, of their atmosphere.
Question 8.
In which part of legumes, nitrogen fixing bacteria are present ? (CCE 2011, 2012)
Answer:
In their root nodules.
Question 9.
What is the function of ozone which is present in the upper level of atmosphere ?
OR
State one use of ozone. (CCE 2011, 2012)
Answer:
Ozone present in the upper layer of atmosphere or stratosphere filters out harmful high energy ultraviolet radiations (100-320 nm) liberating heat in the process that warms up the stratosphere.
Question 10.
Name any one method by which water helps in the formation of soil. (CCE 2011)
Answer:
Passing into cracks of rock, expanding on cooling causing more cracks and widening the previous ones ultimately crushing the rock to form soil particles.
Question 11.
State the role of symbiotic bacteria in nitrogen cycle in nature. (CCE 2011)
Answer:
Symbiotic bacteria live in the root nodules of legumes where they pick up molecular nitrogen from the soil air and convert the same into salts of nitrogen (e.g., ammonium) for use by the host in building amino acids and proteins.
Question 12.
Name two nitrogen compounds obtained by industrial fixation. (CCE 2011)
Answer:
Ammonia, Urea.
Question 13.
Define biogeochemical cycle. Give examples also.
(CCE 2011, 2012)
Answer:
The repeated circulation of biogenetic nutrients or biogeochemicals between the abiotic and biotic components of biosphere is called biogeochemical cycle, e.g, Carbon cycle, Nitrogen cycle, Water cycle, Phosphorus cycle.
Question 14.
Name two biologically important compounds that contain both oxygen and nitrogen. (CCE 2011)
Answer:
Proteins, nucleic acids (DNA, RNA).
Question 15.
Write two methods adopted to control pollution.
(CCE 2011)
Answer:
- Use of CNG instead of gasoline (petrol, diesel) in automobiles,
- Non-discharge of effluents and other pollutants into water bodies and atmosphere by the industries.
Question 16.
Name the disease that can be caused by UV radiations.
(CCE 2011)
Answer:
Skin cancer, eye cataracts.
Question 17.
Name any two water pollutants. (CCE 2011)
Answer:
- Industrial effluents
- Untreated sewage
- Fertilizers, pesticides.
Question 18.
Give two causes of soil erosion. (CCE 2011)
Answer:
Deforestation, overgrazing followed by action of wind or water.
Question 19.
State one cause of depletion of ozone layer. (CCE 2011)
Answer:
Presence of chlorofluorocarbons/halons/nitrogen oxides/ chlorine.
Question 20.
What is soil erosion ? (CCE 2011)
Answer:
Removal of unprotected top soil as through the agency of wind or water is called soil erosion.
Question 21.
Name two factors that make soil/weathering of rocks.
(CCE 2011, 2012)
Answer:
Sun and water.
Question 22.
Mention causes of global warming. (CCE 2011)
Answer:
- Increased concentration of green house gases (e.g, CO2)
- Deforestation.
Question 23.
What is the importance of green house gases present in the atmosphere ? (CCE 2011, 2012)
Answer:
Green house gases do not allow the infra-red rays coming out of earth to escape but to reflect the same to earth making it warm (average temperature 15°C) and suitable for living beings to live comfortably on earth.
Question 24.
Write two biotic components of biosphere ?
(CCE 2011, 2012)
Answer:
Plants, animals, decomposers.
Question 25.
How is oxygen replenished in nature ? (CCE 2011)
Answer:
By photosynthesis.
6CO2 + 6H2O ———-> C6H12O6 + 6O2.
Question 26.
How is average temperature of earth made steady ?
(CCE 2011)
Answer:
Air/atmosphere/green house gases do not allow increase in day temperature and slow down escape of heat during the night.
Question 27.
List any two causes of increase in concentration of CO2 in the atmosphere. (CCE 2011, 2012)
Answer:
- Increasing use of fossil fuels,
- Reduction in vegetation cover.
Question 28.
‘Pesticides reduce soil fertility.’ Give two reasons for your answer. (CCE 2011)
Answer:
- Pesticides kill soil organisms and, therefore, reduce the decomposition of organic matter so that there is lesser release of minerals from the same,
- Decrease in soil organisms like earthworms decreases soil aeration.
Question 29.
What are resources available on earth for life to exist ?
(CCE 2011)
Answer:
Air, water, solar energy, minerals, soil.
Question 30.
Give the name of two air pollutants. (CCE 2011)
Answer:
(i) Suspended particulate matter (SPM).
(ii) Pollutant gases like SO2, nitrogen oxides, methane, carbon monoxide, hydrogen sulphide.
Question 31.
Why is air called breath of life ? (CCE 2011)
Answer:
Air is called breath of life as life cannot exist without air which not only forms a protective transparent envelope or atmosphere around the earth but also provides oxygen (for respiration) and CO2 (for photosynthesis) with a mechanism for their renewal.
Question 32.
How is CO2 fixed in the atmosphere ?
(CCE 2011, 2012)
Answer:
CO2 is being withdrawn continuously from atmosphere by photosynthetic activity of autotrophs. It is returned in nearly equal amount through respiration and combustion.
Question 33.
Which part of solar radiation is absorbed by ozone layer ?
(CCE 2011)
Answer:
Very high energy ultraviolet rays (100 – 320 nm) are absorbed by ozone layer.
Question 34.
What are the constituents of soil ? (CCE 2011)
Answer:
Rock or soil particles (45%), organic matter (humus, 5%), air (25%), water (25%) and different types of microbes (variable).
Question 35.
What is smog ? (CCE 2011)
Answer:
Smog is dark brown or greyish smoky mist that is formed in cold weather due to presence of suspended particles of unburnt carbon, oxides of nitrogen or sulphur in the air. It is suffocating and harmful causing irritation to eyes, nose and lungs besides injuring several internal organs.
Question 36.
Name the group of plants which contain nitrogen fixing bacteria in their root nodules. (CCE 2011, 2012)
Answer:
Legumes.
Question 37.
Name the winds which bring rain in India. (CCE 2011)
Answer:
South-west monsoon and north-east monsoon.
Question 38.
List any two factors that determine the soil type.
(CCE 2011)
Answer:
- Size of soil particles
- Amount of humus/air/water.
Question 39.
Why do lichens not occur in Mumbai whereas they occur in Manali and Ooty ? (CCE 2011)
Answer:
Lichens are very sensitive to air pollution which is quite heavy in Mumbai while air pollution is nearly absent in Manali and Ooty.
Question 40.
What are biodegradable pollutants ? (CCE 2011)
Answer:
Pollutants which can be broken down through the agency of micro-organisms are called biodegradable pollutants, e.g., garbage, sewage.
Question 41.
Give the major source of minerals present in the soil.
(CCE 2011)
Answer:
Rocky matter.
Question 42.
Name two industrial air pollutants. (CCE 2011)
Answer:
- Suspended particulate matter (SPM).
- Oxides of nitrogen and sulphur.
Question 43.
How are winds formed ? (CCE 2011, 2012)
Answer:
- Uneven heating of earth’s surface,
- Differences in heating and cooling of land and water bodies.
Question 44.
Write two methods adopted to control air pollution.
(CCE 2011)
Answer:
- Electronic precipitators,
- Scrubbers.
Question 45.
Name any two green house gases. (CCE 2012, 2013)
Answer:
Carbon dioxide, Methane.
Question 46.
Define the term ‘global warming’ in context to increase in carbon dioxide content of the atmosphere. (CCE 2012)
Answer:
Global warming is the rise in temperature of the surface of earth and its immediate atmosphere due to increase in concentration of green house gases, especially CO2.
Question 47.
What is ozone hole ? (CCE 2012)
Answer:
It is an area of excessive thinning out of ozone in the stratosphere from where high energy UV rays freely pass over to earth, e.g., over Antarctica in spring.
Question 48.
What are CFCs ? (CCE 2012)
Answer:
CFCs are chlorofluorocarbons which have been used extensively in aerosol propellents, refrigerants, formation of foams, spray agents, etc. They are strong ozone depleting substances (ODS).
Question 49.
Name the atmospheric gas that leads to global warming .
(CCE 2012)
Answer:
Carbon dioxide.
Question 50.
Suggest a way of bringing underground water to the surface of earth. (CCE 2012)
Answer:
By means of tube wells, dug wells, hand pumps.
Question 51.
State the percentage of nitrogen gas in the atmosphere.
(CCE 2012)
Answer:
78%.
Question 52.
Mention any one effect of increase in the amount of green house gases in the atmosphere. (CCE 2012)
Answer:
Rise in temperature (global warming).
Question 53.
“Water is essential for life”. Justify the statement.
(CCE 2012)
Answer:
Water is solvent, reaction medium and transport medium of biomolecules constituting living matter.
Question 54.
The large scale deforestation should be stopped. List two reasons to justify. (CCE 2012)
Answer:
Large scale deforestation should be stopped immediately as it is
- Raising CO2 concentration in the atmosphere resulting in global warming.
- Causing floods and soil erosion.
Question 55.
Name the compounds responsible for hole in ozone layer of atmosphere. (CCE 2012)
Answer:
Chlorofluorocarbons, halons, carbon tetrachloride, methyl bromide, methyl chloroform, nitrogen oxides, chlorine.
Question 56.
Name any two pollutants produced by burning of coal and petroleum. (CCE 2012)
Answer:
Suspended particulate matter (SPM), pollutant gases (e.g., sulphur dioxide, nitrogen oxides), hydrocarbons.
Question 57.
Mention two factors that influence the pattern of winds.
(CCE 2012)
Answer:
- Difference in heating and cooling of land and water bodies.
- Rotation of earth.
- Inclination to sun.
Question 58.
List two human activities that would lead to air pollution.
(CCE 2012)
Answer:
- Burning of fossil fuels.
- Gaseous emissions from industries.
Question 59.
Name two free living nitrogen fixing bacteria.
(CCE 2012)
Answer:
Azotobacter, Beijerinkia.
Question 60.
Name the process by which carbon is incorporated into life forms like plants. (CCE 2012)
Answer:
Photosynthesis.
Question 61.
Name the organism sensitive to SO2 in air. (CCE 2012)
Answer:
Lichens
Question 62.
Why do plants need nitrogen ? (CCE 2012)
Answer:
For building up amino acids, proteins, nucleotides, nucleic acids, enzymes, alkaloids and some vitamins.
Question 63.
Name any two physical agents that cause weathering of rocks. (CCE 2012)
Answer:
Atmospheric changes (heating-cooling, wetting-drying, frostraction),- mechanical forces (abrasion by sand, rain, hail,
rolling stones, wave action).
Question 64.
What is the conversion of ammonia to nitrates called ?
(CCE 2012)
Answer:
Nitrification.
Question 65.
List two activities of man which lead to environmental pollution. (CCE 2012)
Answer:
- Burning of fossil fuel
- Passage of sewage or municipal water into water bodies.
Question 66.
Name two chemicals present in living organisms having carbon, hydrogen and oxygen as main constituents.
(CCE 2012)
Answer:
Carbohydrates, fats. They are food articles which function as storage and source of energy.
Question 67.
Frozen water between cracked rocks causes the cracks to widen. How? (CCE 2013)
Answer:
Frozen water occupies more volume than liquid water. It exerts a large mechanical force on sides of rock cracks which, therefore, widen.
Question 68.
What does hydrosphere consist of ? (CCE 2013)
Answer:
Hydrosphere or water component of earth includes all the water present in, on and around the earth.
Question 69.
State any one difference between oxygen and ozone.
(CCE 2013)
Answer:
Oxygen is diatomic (O2) while ozone is triatomic (O3)
Question 70.
Name two elemental forms of carbon found on the earth ?
(CCE 2013)
Answer:
Graphite, Diamond.
Question 71.
State the percentage of nitrogen gas in the atmoshpere.
(CCE 2013)
Answer:
78%
Question 72.
Name the poisonous gas formed from oxygen gas in the atmosphere ? (CCE 2013)
Answer:
Ozone.
Question 73.
What is meant by the term biosphere ? (CCE 2013)
Answer:
Part of earth which supports life is called biosphere.
Question 74.
Name the factor responsible for rainfall patterns in India.
(CCE 2013)
Answer:
South-west monsoon and to a smaller extent north-east monsoon.
Question 75.
Name the stages of the life cycle of aquatic animals which are affected by change in temperature. (CCE 2014)
Answer:
Egg (hatching), larvae and young animals.
Question 76.
Write the name of triatomic molecule of oxygen.
(CCE 2014)
Answer:
Ozone (O3).
Question 77.
State the temperature range on the surface of moon.
(CCE 2014)
Answer:
-190°C (dark period) to 110°C (light period).
Question 78.
Mention the direction in which air flows at night in coastal areas. (CCE 2014)
Answer:
From land to sea.
Question 79.
Alongwith the natural resources available on the earth, what else is required to meet the basic requirement of all life forms on the earth ? (CCE 2014)
Answer:
Solar energy.
Question 80.
Name the gas produced when glucose molecules breakdown using oxygen. (CCE 2014)
Answer:
Carbon dioxide (CO2).
Question 81.
Name two natural resources available on earth.
(CCE 2014)
Answer:
- Renewable, exhaustible, e.g., forests,
- Non-renewable exhaustible, e.g., fossil fuels.
Short Answer Questions (2 Marks)
Question 1.
Mention two ways of fixing carbon dioxide.
(CCE 2011)
Answer:
- Photosynthesis, also called carbon assimilation, by autotrophic plants,
- Formation of shells and components of skeleton in marine animals.
Question 2.
How temperature/sun and water help in soil formation ?
(CCE 2011, 2013)
Answer:
- Temperature: Rocks expand on being heated up in the sun and contract on cooling during night. This alternate heating and cooling produces cracks that ultimately break up rocks into smaller pieces and particles.
- Water: Water entering cracks causes the cracks to widen on being frozen resulting in more cracks and breaking of rocks. Water carrying suspended particles flowing over the rocks corrodes the surface of the latter removing more particles that collect to form soil.
Question 3.
Give reason for the following :
(i) We are lucky that ozone is not stable near the earth’s surface,
(ii) The combustion of fossil fuels increases the amount of suspended particles in air. (CCE 2011)
Answer:
(i) Ozone occurring near the surface of the earth is a strong oxidant causing corrosion of articles, hardening of rubber, respiratory distress and internal haemorrhages. Fortunately, it is not stable forming oxygen and oxides.
(ii) Combustion of fossil fuels is never one hundred percent complete. A lot of unburnt carbon particles or hydrocarbons come out in fumes or exhausts and form suspended particles in the air.
Question 4.
(i) Why is water necessary for all living organisms ? Mention any two points in support of your answer.
(ii) Water is known as “wonder liquid”. Justify this statement by giving any two reasons.
(CCE 2011, 2012)
Answer:
(i) Necessity of Water:
- Component: Water is a major component (60-90%) of living matter and is a general solvent for various biochemicals which also react in the medium of water.
- Transport: Water is a medium of transport in the body of living organisms.
- Buffer: It also functions as a temperature buffer.
(ii) A Wonder Liquid: Water is liquid between 0° and 100°C. It forms solid ice below 0°C and vapours above 100°C. It becomes available at far off places as rain, rivers and springs. Underground water is source of fresh water at many places.
Question 5.
(i) Define the term smog,
(ii) Name two types of diseases caused by regularly breathing the polluted air.
(CCE 2011)
Answer:
(i) Smog is dark brown or greyish smoky mist that is formed in cold weather due to presence of suspended particles of unburnt carbon, oxides of nitrogen or sulphur in the air. It is suffocating and harmful causing irritation to eyes, nose and lungs besides injuring several internal organs.
(ii) Diseases. Pneumoconiasis, Asthma, Bronchitis, damage to liver, kidneys and cancer.
Question 6.
What is green house effect ? How is it caused ?
(CCE 2011, 2012, 2014)
Answer:
Green house effect is keeping an area warm by allowing the solar radiations to pass in but preventing long waves to escape due to presence of radiatively active gases and glass panes.
Certain gases present in the atmosphere of the earth provide a green house effect over earth, keeping it warm with an average temperature of 15°C, e.g., C02, methane, chlorofluoro-carbons, nitrous oxide.
Question 7.
Give the chemical formula of Ozone ? What is its role in atmosphere ? (CCE 2011)
Answer:
O3. Ozone is good ozone in stratosphere where it filters out very harmful ultraviolet radiations (100-320 nm) coming from sun. It is bad ozone near the surface of earth because of its highly oxidising nature.
Question 8.
(a) Why do terrestrial forms require fresh water ?
(b) Give two examples where fresh water can be found in the frozen form on earth. (CCE 2011)
Answer:
(a) The osmotic concentration of terrestrial forms is low. They can neither store nor eliminate high amounts of dissolved salt present in sea water. Therefore, they require fresh water.
(b) Ice caps over poles, Glaciers.
Question 9.
(a) What is soil erosion ? State any one way by which it can be prevented.
(b) What is humus ? What is the role of earthworms in increasing the quantity of humus. (CCE 2011)
Answer:
(a) Soil Erosion: Removal of unprotected top soil as through the agency of wind or water is called soil erosion.
Soil erosion can be prevented by covering the soil with vegetation.
(b) Semidecayed organic matter present in the soil is called humus. Earthworms bring about fragmentation of organic remains and add humus and mineral rich worm castings in the soil.
Question 10.
What is atmospheric fixation of nitrogen ? (CCE 2011)
Answer:
It is the formation of oxides of nitrogen from atmospheric dinitrogen (N2) in the presence of energy from electric storms, lightning and high energy ultraviolet rays. Nitrogen oxides dissolve in rain water as nitrous and nitric acids. They pass down to soil and water bodies forming nitrites and nitrates.
Question 11.
The heaps of solid waste are a menace. Give two reasons.
(CCE 2011)
Answer:
Solid waste is discarded solid material dumped over soil, e.g., municipal waste, industrial waste, mining waste.
- Municipal and industrial waste often gives a strinking bad smell.
- Toxic chemicals present in the solid waste often seep into ground water making the same unfit for human consumption.
- It makes the area of earth useless and infertile.
Question 12.
How is addition of undesirable substances and change in temperature affect the water life ? (CCE 2011, 2013)
Answer:
- Undesirable Substances: Fertilizers, pesticides, industrial effluents, municipal waste water when passed into water bodies cause eutrophication that later on kills most of the aquatic life due to depletion of oxygen and presence of toxic chemicals.
- Temperature: A change in temperature affects oxygen concentration. A high temperature reduces O2 content which reduces the rate of decomposition of organic matter, causes asphyxiation of aquatic animals, non-hatching of eggs and killing of larvae.
Question 13.
Name two measures that can be taken to reduce water pollution. (CCE 2011)
Answer:
- Treatment of municipal waste water before discharging it into water body.
- Treatment of industrial effluents before passing it into municipal sewers or water body.
Question 14.
(i) What is the role of atmosphere in climate control ?
(ii) What is the percentage of nitrogen and oxygen in the air ? (CCE 2011)
Answer:
(i) Atmosphere helps to maintain the climate of a place (which depends upon inclination and air currents) by acting as temperature buffer, preventing rise of temperature during the day and excessive cooling during the night. For this, the atmosphere is a bad conductor of heat.
(ii) Nitrogen – 78%, Oxygen – 21%.
Question 15.
Mention any two human activities which are responsible for water pollution. (CCE 2011)
Answer:
- Runoff of fertilizers and pesticides applied to crop fields.
- Passage of municipal waste water and industrial effluents into water bodies.
Question 16.
Differentiate between biodegradable and non- biodegradable pollutants. (CCE 2011)
| Biodegradable Pollutants | Non-biodegradable Pollutants |
| 1. Nature. They are biological in origin which can be degraded by decomposer microo-rganisms. | 1. They are non-biological in origin which cannot be degraded by micro-organisms. |
| 2. Accumulation. They do not accumulate | 2.They tend to accumulate and even show biomagnification. |
| Ex. Cowdung, Garbage,Sewage. | Ex. Plastic, Mercury,DDT. |
Answer:
Biodegradable Pollutants Non-biodegradable
Question 17.
(a) Name the process that returns oxygen to the atmosphere.
![]()
(b) Write the condition responsible for poor visibility in cold weather. (CCE 2011)
Answer:
(a) Photosynthesis is the metabolic process that brings about carbon assimilation and releases oxygen, energy
(b) In cold weather, water vapours condense over suspended particulate matter (dust, unburnt carbon) present in the air resulting in foggy conditions and poor visibility.
Question 18.
List two measures of conserving soil fertility.
(CCE 2011)
Answer:
Conservation of soil fertility is the maintenance of ability of the soil to support crops and vegetation. It is carried out by
- Crop rotation
- Green manuring
- Regular addition of farmyard manure.
Question 19.
What causes acid rain ? Mention any damage caused by it on living organisms. (CCE 2011)
Answer:
Acid rain is rainfall with a pH of less than 5. It is caused by the excess emission of SO2 and nitrogen oxides. The gases dissolve in water forming nitric acid and sulphuric acid. Acid rain causes chlorosis, defoliation, stunting and dieback of plants. It causes skin and lung irritation. Marble structures, metallic articles, jewellary, textiles and paintings are also spoiled by acid rain.
Question 20.
Which symbiotic life forms can grow on stones and help in formation of soil ? Write the mode of their action for making soil from rocks. (CCE 2011)
Answer:
Lichens are dual or composite forms which can grow on bare rocks and bring about biological weathering. The lichens secrete organic acids over the rock surface corroding the same and producing small crevices. Later on, the crevices grow into cracks producing soil.
Question 21.
Explain the role of atmosphere as a blanket. List the factors deciding the rainfall pattern. (CCE 2011)
Answer:
- Atmosphere as Blanket: Atmosphere is a bad conductor of heat. It does not allow over heating during daytime or excessive cooling during night. Instead, it helps to maintain the average temperature of the earth.
- Factors Deciding Rainfall Patterns :
- Availability of water vapours,
- Areas of low pressure or depression
- Prevailing wind
- Condensation of water vapours.
- Direction of mountains.
Question 22.
How do forests play an important role in maintaining water cycle ? (CCE 2011)
Answer:
Forests, being large areas of vegetation, transpire a large amount of water in the vapour form. The water vapours being lighter, rise in the air, cool down and form tiny water droplets around air borne particles producing clouds. In contact with cold air, clouds produce rain. Rain falling over a forest area mosdy percolates into the soil from where the same is absorbed by the forest plants.
Question 23.
What are green houses ? (CCE 2011)
Answer:
Green houses are enclosures with glass panes which allow the solar radiations to pass inwards but prevent the escape of infra-red heat waves. As a result, green houses remain warm. They are useful in growing tropical plants in colder areas.
Question 24.
List any four activities you think would lead to air pollution. (CCE 2011)
Answer:
- Excessive use of fossil fuels like coal and petroleum.
- Deforestation,
- Smoke from industries,
- Smoke from automobiles,
- Mining activities.
Question 25.
What is top soil ? Mention any two factors that decide which plants will thrive on that soil. (CCE 2011)
Answer:
Top soil is the upper mineral and humus rich layer of soil that supports vegetation.
Factors:
- Depth of top soil,
- Nutrient and humus content of soil,
- Soil particles,
- Soil pH.
Short Answer Questions (3 Marks)
Question 1.
(a) “The flow of energy is unidirectional whereas the biogeochemical transfer is cyclic.” Explain why.
(b) Justify the statement, “The nitrogen cycle is supposed to be an ideal cycle in the biosphere.” (CCE 2011)
Answer:
(a) Energy is regularly entering the biosphere from the sun. It is trapped by the producers from where it passes to herbivores and then to carnivores. The energy present in organic remains passes into decomposers. At every step of this transfer there is a lot of dissipation of energy which ultimately leaves the biosphere.
Biogeochemicals are components of biosphere which neither came from outside nor are lost. They are picked / up by producers, passed to consumers and returned to producers by the activity of decomposers. Therefore, flow
(b) Amount of nitrogen remains almost constant in the atmosphere as well as soil. No nitrogen is lost. It is because a part of nitrogen is being fixed regularly in the atmosphere while an equal part of nitrogen is being added to atmosphere through denitrification. Nitrogen salts are also undergoing biogeochemical cycling.
Question 2.
List any three ways to control soil pollution. (CCE 2011)
Answer:
- Minimum or no use of chemical fertilizers, insecticides and pesticides.
- Dumping of solid wastes only in sanitary fill.
- Proper disposal of garbage in composting, biogas plants and incineration.
Question 3.
“It is necessary to conserve natural resources.” Explain.
(CCE 2012)
Answer:
Natural resources (e.g., air, water, soil, forests, minerals, fossil fuels) are earth resources which are being used by human beings for meeting their requirements. However, due to their indiscriminate and excessive use, there is already shortage of these resources and causing harm to our welfare. Therefore, there is an urgent requirement of their conservation by controlling their use and measures to increase their availability through
- Rain water harvesting.
- Afforestation and reforestation along with controlled grazing and cutting.
- Restoration of soil fertility and prevention of soil erosion.
- Reuse, recycling and substitution of minerals.
- Judicious use of fossil fuels and finding of alternate sources of energy.
Question 4.
(a) What are different ways in which water gets polluted ?
(b) How does it affect life forms ? (CCE 2012)
Answer:
(a) Water pollution is caused by passage of
- Untreated or partially treated sewage.
- Runoff from argicultural fields treated with fertilizers and pesticides.
- Passage of untreated industrial effluents and hot water.
- Oil spills.
(b) Water pollution causes :
- Spread of water borne diseases (e.g., typhoid, cholera, jaundice)
- Eutrophication or nutrient enrichment followed by algal blooms, death of aquatic animals and organic loading leading to foul smell and taste.
- Industrial effluents having toxic chemicals causing diseases as minamata disease, itai itai disease, plumbism, methaemoglobinemia, fluorosis, black foot disease, cancer.
Question 5.
(a) How is soil formed ?
(b) What is soil erosion ?
(c) Write two steps to control soil erosion. (CCE 2012)
Answer:
(a) Soil Formation. Soil is formed by weathering (pulverisation) of rocks through physical (sun, water, air), chemical (hydration, oxidation, reduction) and biological (lichens, mosses, herbs) factors followed by humification.
(b) Soil Erosion: Removal of unprotected top soil as through the agency of wind or water is called soil erosion.
(c) Control of Soil Erosion,
- Afforestation.
- Wind breaks.
- Step cultivation on hills.
Question 6.
(a) List the causes that affect life forms found in water bodies in various ways.
(b) Name the element present in coal other than carbon that releases harmful gases during combustion of coal.
(CCE 2012)
Answer:
(a) Water pollutants like untreated sewage, industrial effluents, fertilizer and pesticide rich run off from agricultural fields. .
(b) Sulphur, producing sulphur oxides (e.g., S02) during combustion of coal.
Question 7.
(a) What are the two different states in which water is formed during the water cycle ?
(b) What is the major source of fresh water in the city/town/village where you live ?
(c) Do you know of any activity which may be polluting this water source ? (CCE 2012)
Answer:
(a) Water vapours, liquid water.
(b) The major source of fresh water is ground water in my locality. It is pumped out by tube wells.
(c) It is being polluted by leaching of heavy metals e.g., Nickel from industrial effluents.
Question 8.
How is ‘soil formation different from ‘soil erosion ? Write two factors responsible for each one of them. (CCE 2012)
Answer:
Soil formation (Paedogenesis) is formation of soil from rocky crust of earth while soil erosion is removal of top soil. Factors for Soil Formation: Sun (heating and cooling), water (wetting and drying, freezing, abrasion).
Factors for Soil Erosion. Absence of vegetation cover, heavy rain or strong wind.
Question 9.
(a) “An increase in temperature of water bodies would lead to water pollution”. Explain.
(b) Suggest any two methods to prevent water pollution.
(CCE 2012)
Answer:
(a) Increase in Temperature: A change in temperature affects oxygen concentration. A high temperature reduces O2 content which reduces the rate of decomposition of organic matter, causes asphyxiation of aquatic animals, non-hatching of eggs and killing of larvae.
(b) Prevention of Water Pollution:
- Sewage: Only fully treated sewage water should be allowed to pass into water body.
- Industrial Effluents: They should not be allowed to flow into water body without treatment.
Question 10.
(a) Ozone is poisonous and is found in the upper atmosphere of earth. Yet the world is worried about its depletion. Explain why ?
(b) Write any two methods to control ozone layer depletion.
Answer:
(a) Ozone present in the upper layer of atmosphere or stratosphere filters out harmful high energy ultraviolet radiations (100-320 nm) liberating heat in the process that warms up the stratosphere.
(b)
- Replacing chlorofluorocarbons and halons with ozone friendly chemicals.
- Use of jet fuel with little nitrogen.
Question 11.
(a) What are various components of soil.
(b) “Fertilizers and pesticides lead to soil pollution.” List two reasons to justify the statement. (CCE 2012, 2013)
Answer:
(a) Soil consists of mineral matter (45%), organic matter (5%), air (25%), water (25%) and variable number of soil organism. Mineral matter consists of gravel (size 2-10 mm), sand (0.02-2.0 mm), silt (0.002-0.02 mm) and clay (below 0.002 mm) Organic matter is in semidecayed form and is called humus.
(b) Both fertilizers and pesticides cause soil pollution by
- Killing or inhibiting the growth of soil micro- organisms.
- Destruction of soil crumbs.
- Their passage into crops and ground water causing toxic effects over humans and animals (e.g., producing methaemoglobin).
Question 12.
Name four main processes that are part of water cycle in nature. (CCE 2012)
Answer:
- Vaporisation
- Cloud formation
- Rain
- Infiltration and run-off.
Question 13.
What is smog ? List any two harmful effects of smog.
(CCE 2012)
Answer:
(a) Smog is dark brown or greyish smoky mist that is formed in cold weather due to presence of suspended particles of unburnt carbon, oxides of nitrogen or sulphur in the air. It is suffocating and harmful causing irritation to eyes, nose and lungs besides injuring several internal organs.
(b) Harmful Effects,
- Allergy, asthma, bronchitis and suffocaton in human beings and animals.
- Silvering, glazing and necrosis in plants.
Question 14.
Explain the formation of sea breeze and land breeze in coastal areas. (CCE 2012)
Answer:
Sea Breeze. It is flow of cooler breeze from sea towards the land during the day time. During day time, land gets heated faster. Air above land heats up, rises and creates an area of low pressure. Air above sea is cooler and has higher pressure. It moves towards land.
Land Breeze. During night, land cools rapidly while sea water remains warmer. Air above sea is warmer, rises and develops low pressure. Air above land is cooler and has higher pressure. It flows towards sea.
Question 15.
State any two sources of water pollution. Which type of pollution affects the breeding of aquatic animals.
(CCE 2012)
Answer:
Sources of Water Pollution,
- Sewage
- Fertilizers and pesticides
- Industrial effluents.
Temperature: Increase or decrease of temperature of water body affects breeding of most aquatic animals. A high temperature reduces the amount of oxygen dissolved in water.
Question 16.
(a) How does the atmosphere act as a blanket.
(b) The moon which is about the same distance from the sun as that of earth but still there is no atmosphere on moon. (CCE 2012)
Answer:
(a) Atmosphere as Blanket: Atmosphere is a bad conductor of heat. It does not allow over heating during daytime or excessive cooling during night. Instead, it helps to maintain the average temperature of the earth.
(b) Moon Has No Atmosphere: Atmosphere is maintained by gravity and circulation of its components. Moon has only 1/6 gravity as compared to earth. It does not hold any atmosphere. There is no living organisms which can produce and utilise camponents of atmosphere.
Question 17.
Name the constituents of abiotic component of the biosphere. Explain their major role in biosphere. (CCE 2012)
Answer:
Air, water and soil.
- Air/Atmosphere: It functions as a blanket, source of O2 and CO2, medium of dispersal, formation movement and precipitation of water vapours.
- Water: Water is a component of living beings (60-90%), solvent, transport medium, reaction medium for biomolecules, temperature buffer and bringing turgidity to living cells.
- Soil: It provides anchorage, water and minerals to plants, a place for microorganisms to dispose off organic remains and release of minerals.
Question 18.
(a) What is soil pollution ? What factors are responsible for it ?
(b) Suggest two methods of preventing or reducing soil pollution. (CCE 2012)
Answer:
(a) Soil Pollution. It is adverse alteradon in the soil caused by removal or addition of substances which reduce its fertility and quality.
(b) Factors. Addition of raw manure, excess fertilizers, pesticides, faulty irrigation and dumping of wastes as well as industrial effluents.
(c) Control of Soil Pollution.
- Minimum or no use of chemical fertilizers, insecticides and pesticides.
- Dumping of solid wastes only in sanitary fill.
- Proper disposal of garbage in composting, biogas plants and incineration.
Question 19.
Write one appropriate word for statements (1—3) given below and briefly explain them.
- The process by which carbon is incorporated into life forms.
- The process by which nitrates are converted into free nitrogen.
- The cyclic flow of nutrients like nitrogen and oxygen between nonliving environment and living organisms.
(CCE 2012)
Answer:
- Photosynthesis: CO2 is taken up by plants from their environment and changed into organic form. Energy is taken from sun. Hydrogen is taken from water.
- Denitrification: Some bacteria present in water-logged soils take oxygen from nitrates for their activity. This changes nitrates into gaseous nitrogen.
- Biogeochemical Cycling: Biogeochemicals or nutrients are taken up by organisms for their structure and functioning. They are released later on when organic remains are decomposed. The same are picked up again by the organisms.
Question 20.
(a) Name two elemental forms in which carbon occurs.
(b) Name any two forms in which carbon is found in combined state with other elements in nature.
(c) Briefly explain how it can be said that all life forms are based on carbon containing molecules. (CCE 2012)
Answer:
(a) Elemental Forms. Diamond, Graphite.
(b) Combined State. Carbon dioxide, Carbohydrate.
(c) Life Forms. All life forms contain protoplasm, energy storing and energy liberating organic substances and enzymes for catalysing various metabolic reactions. All these are organic substances made of carbon, e.g, proteins, enzymes, carbohydrates.
Question 21.
(a) What is the role of bacteria in nitrogen cycle ?
(b) What happens to nitrate once it enters the plant ?
(CCE 2012)
Answer:
(a) Role of Bacteria,
- Biological nitrogen fixation, e.g., Azotobacter, Rhizobium.
- Decomposition, ammonification and nitrification, e.g., Bacillus ramosus, Nitrosomonas, Nitrobacter.
- Denitrification, e.g., Pseudomonas.
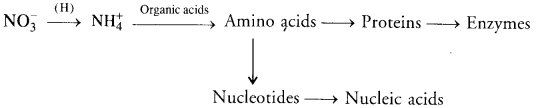
(b) Nitrogen Assimilation. Nitrate absorbed by plants is first reduced to ammonia level. Ammonia combines with organic acids to produce amino acids from which proteins and nucleotides are formed.
Question 22.
Give reason :
(a) Presence of high levels of suspended particles in the air is harmful.
(b) A sudden change in the temperature of water body is dangerous for aquatic life.
(c) The process of nitrogen fixation does not take place in the presence of oxygen. (CCE 2012)
Answer:
(a) Some of the suspended particles present in the air enter our lungs, reach alveoli and cause pulmonary ailments like asthma, bronchitis, emphysema, lung fibrosis, pneumoconiosis.
(b) Change in temperature changes the amount of oxygen dissolved in water and disturbs the breeding of aquatic animals.
(c) The enzyme for nitrogen fixation called nitrogenase is sensitive to oxygen as it is specialized to combine hydrogen with nitrogen.
Question 23.
What makes the biosphere dynamic but stable ?
(CCE 2012)
Answer:
The composition of atmosphere, the extent of hydrosphere and lithosphere remain stable in the biosphere. It is because of regular circulation of their constituents among different components and between biotic and abiotic parts of the biosphere. The phenomenon is called biogeochemical cyclling.
Question 24.
Design an activity to show how convective currents are set up in the air and what is the nature of these currents ?
(CCE 2012)
Answer:
Demonstration of Convectic Currents
Apparatus: A beaker or wide mouthed bottle, a candle, box of matches, incense stick (agarbatti).
Working: Fix a burning candle in a beaker or wide mouthed bottle. Light an incense stick. Take it first to edge of the beaker, then half way towards middle and finally above the candle flame.
Results: When kept near the edge, smoke from incense stick will move towards the flame. This will also occur when it is halfway towards the flame. However, when the incense stick is kept above the flame, the smoke will rise up. In all cases smoke flows towards the area of low air pressure.
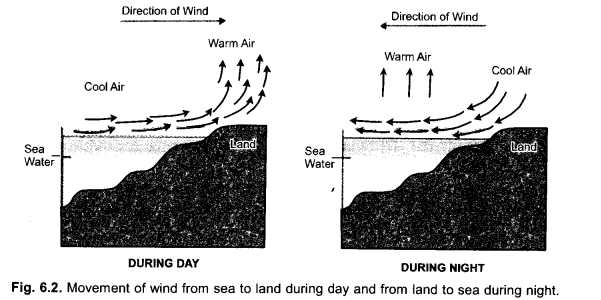
During night, reverse flow of air occurs. Land cools down rapidly. Air over it becomes cooler. Sea water cools slowly. Air above it is hotter with lower air pressure as compared to air pressure over land. Therefore, cooler air present over land moves towards sea.
Factors Influencing Movements of Air. Depending upon the difference in energy levels and air pressure, there occurs diverse type of atmospheric phenomena like breeze, wind, storm, thunderstorm, monsoon rains, cyclones, etc. The factors controlling these phenomena are
- Uneven heating of land in different parts of earth
- Differences in heating and cooling of land and water.
- Barrier of mountains
- Rotation of earth.
Question 25.
State two consequences of global warming. (CCE 2012)
Answer:
- Melting of polar ice and glaciers will raise the water level of sea submerging many low level coastal areas.
- Atmosphere will shrink due to cooling caused by reduced passage of long wave radiations.
- Tropics will be converted into arid areas while floods will occur in higher latitudes.
Question 26.
Explain the role of atmosphere in keeping climate control.
(CCE 2012)
Answer:
Atmosphere helps in maintaining the climate of an area by retaining the heat over the area as it is a temperature buffer. There is neither excessive heating during day nor excessive cooling during night. The average temperature of a place remains steady as per inclination of the area towards sun.
Question 27.
Mention the importance of soil. How is it depleted? Write two ways to replenish soil. (CCE 2012)
Answer:
(a) Soil provides anchorage, minerals and water to terrestrial plants. It is also residence of microorganisms involved in decomposition of organic remains and release of minerals.
(b) Soil Depletion. It occurs through soil erosion, water logging and salination.
(c) Replenishment of Soil. Providing a vegetation cover, correction of water logging and salination, crop rotation and contour bunding.
Question 28.
Explain the following terms :
- Ammonification
- Nitrification
- Biosphere.
(CCE 2012)
Answer:
- Ammonification: It is breakdown of proteins and deamination of amino acids to release ammonia.
- Nitrification: It is oxidation of ammonia released through ammonification to form nitrites and nitrates.
- Biosphere: It is living mande of earth comprising lower atmosphere, lithosphere and hydrosphere where living beings are present.
Question 29.
How has industrialisation led to air pollution ? Explain in three points. (CCE 2012)
Answer:
- Processing industries release a lot of air pollutants (e.g., cotton dust, iron mill dust, flour mill dust, cement industries, asbestos industry) that cause pneumoconiosis and other pulmonary problems.
- Fuel. Industries consume coal and other fossil fuels which send out smoke, pulluting gases and hydrocarbons,
- Chemical industries send out toxic chemicals into air that have a harmful effect on plants, animals and humans,
Question 30.
How is smog formed ? What does it indicate ? (CCE 2012)
Answer:
Smog is opaque fog which is formed due to noncirculation of air that results in accumulation of suspended particulate matter, pollutant gases, hydrocarbons and water vapours.
It indicates high level of pollution in the area due to industries and vehicular traffic.
Question 31.
Two similar plastic trays filled with soil and manure are taken. In tray A mustard seeds are sown and watered for 4-5 days until they germinate into seedlings and the seedlings grow into small plants. Watering of the tray A is stopped for the next 2-3 days and the small plants are allowed to grow. Trays A and B are placed over a brick in a tilted position. Now both the trays are watered with equal amount of water using a water can.
(a) Name the natural phenomenon indicated by tray B.
(b) Less of water flowing out is indicated by tray A. Give reason.
(c) Why is the top layer of soil considered the most important layer ? (CCE 2012)
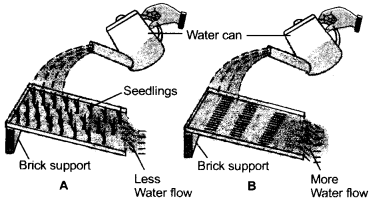
Answer:
(a) Run off, which is high in soil not covered by vegetation.
(b) Most of the water is absorbed by soil as the latter is porous due to presence of plants. Run off is little.
(c) Top soil is important because it contains humus, air and decomposer microorganisms. Roots of most plants are restricted to it.
Question 32.
Explain the role played by lichens, moss and trees in the soil formation. (CCE 2012)
Answer:
Soil is formed through two processes of weathering and humification.
It is pulverisation of rocks or breaking of rocks into fine particles. There are three types of weathering — physical (atmospheric changes and mechanical forces), chemical and biological. Sun, water, wind and living organisms perform them.
- Sun: It causes expansion of rocks by heating. Cooling causes their contraction. Different parts of rocks expand and contract at different rates. Uneven expansion and contraction produces cracks leading to fragmentation of rocks.
- Water:
- Wetting and Drying: Certain rock components can pick up and lose moisture. They undergo swelling and contraction resulting in fragmentation of rocks.
- Frost Action: Water seeping in cracks would swell up and exert a great pressure if it freezes due to low temperature. The rock would undergo fragmentation.
- Abrasion: Running water carrying rock fragments would break and grind rocks occurring in the pathway. Rain and hail also cause rock breaking.
- Wind: Dust and fine sand carried by wind cause abrasion of the rock surface when wind strikes the same.
- Living Organisms: Lichens secrete chemicals to dissolve minerals from the rock surface. This produces crevices where dust collects. Mosses grow there. They cause deepening of crevices and development of small cracks. Roots of short lived plants widen these cracks. Roots of larger plants cause fragmentation of rocks by entering the cracks and growing in size.
Question 33.
How are clouds formed and bring us rain ? Explain briefly. (CCE 2012)
Answer:
Clouds are wet air masses that float in the direction of prevailing wind. They develop when water vapours are formed in large number. There is evaporation from the surface of water bodies and wet areas due to their heating during the daytime. Plants also give out water vapours in transpiration while animals do so in exhaled air and perspiration. Air also heats up during daytime. The hot air along with water vapour rises up. At a height, air expands and becomes cool. Cooling causes the water vapours to condense. Suspended particles of dust and other materials function as nuclei around which water vapours condense. When a large wet air mass collects, cloud is formed.
Clouds move along the prevailing direction of wind. Wherever they meet cool air or high mountains, further condensation of water droplets change the same into rain.
Question 34.
How do modern farming practices bring about conversion of fertile soil to barren soil ? Explain briefly (CCE 2012)
Answer:
- Clean Tilling: The exposed top soil layer gets dried and it is blown away by wind.
- Fertilizers: They are chemical formulations which are added to soil in order to provide specific nutrients to crop plants.
- Pesticides: They are broad spectrum formulations which are. used to kill pests and weeds. Some of these pesticides are slow to degrade. They undergo biomagnification and prove harmful to higher forms of life, e.g., chlorinated hydrocarbons.
- Excessive Irrigation: Faulty irrigation causes water logging and soil salination. Both of them reduce crop yield drastically.
Therefore, indiscriminate use of fertilizers, pesticides and faulty irrigation can turn fertile crop fields into barren areas. It is important to adopt sustainable practices like organic farming and sprinkler irrigation.
Question 35.
What is soil ? How is it formed ? State the major factor that decides the structure of soil. What role does it play ?
(CCE 2012, 2013)
Answer:
Soil: It is upper weathered and mineral containing part of earth’s crust which supports plant life and contains numerous organisms and organic remains.
Soil Formation: Soil is formed by weathering (pulverisation) of rocks through physical (sun, water, air), chemical (hydration, oxidation, reduction) and biological (lichens, mosses, herbs) factors followed by humification.
Major Factor: Average particle size determines the structure of soil, e.g., clayey, loam, sandy. Soil particles determine j mineral availability, soil hydration and soil aeration.
Question 36.
Geeta visited her relatives living in a village. She found that many villagers were suffering from water borne disease like cholera and jaundice. She also noticed that the pond in the village used as a water source did not have any aquatic plants and animals.
- What could be the reason for mass illness ?
- Write two reasons for the pond to have been devoid of life. (CCE 2013)
Answer:
- Reason for Mass Illness. Contamination of pond water with sewage,
- Reasons for Absence of Aquatic Plants and Animals,
- Loading of water with sewage could cause increased activity of decomposers which deplete the pond of its dissolved oxygen.
- Absence of oxygen kills all the aquatic animals and plants.
Question 37.
Rajesh lived in a village which was pollution free. He visited j an industrial city during winter and found the visibility very poor. Many children were showing signs of allergy. What could have been the reason. Suggest any two ways which could have helped to improve the situation.
(CCE 2013)
Answer:
The industrial city was engulfed in smog. Smog contains components (e.g., smoke particles, S02, H2S, hydrocarbons, etc) which are injurious to human beings, causing allergy, suffocation and irritation.
- Switch over from coal-fired engines to gasoline and electric engines in industries,
- Tall chimneys and passing the smoke through precipitators and scrubbers,
- Strict enforcement of pollution control norms over vehicles.
Question 38.
(a) Where is the ozone layer found ?
(b) What is ozone hole and how is it caused ?
(c) State its harmful effects.
(CCE 2013, 2014)
Answer:
(a) Ozone layer is ozone (O3) rich upper part of atmosphere called stratosphere. It lies at a height of 23-25 km above equator and 11-16 km above poles.
(b) Ozone Hole. Ozone hole is area of excessive thinning of ozone in the stratosphere. It is mostly found over Antarctica and to a lesser extent over north pole during their spring times. Cause. Ozone depletion occurs due to certain chemicals called ozone depleting substances (ODS), e.g., chlorofluorocarbons (CFCs), halons, nitrogen oxides, chlorine. They react with 03 and change it to oxygen state,
(c) Harmful Effects. Thinning of ozone layer results in passing very high energy ultraviolet rays (100—320 nm) to the earth. They cause skin cancer, harm eye sight, weaken immune system, cause harmful mutations and reduce crop yield.
Question 39.
Describe the structure of a simple glass house mentioning the climatic conditions in which they are used. Name any two green house gases. (CCE 2013)
Answer:
Green house is an enclosure having glass panes in the direction where solar radiations fall inside. Green houses are built in cold climates for growing tropical plants even in winters. It is because the interior of glass house remains warm because glass panes (and water vapours, CO2 in the enclosure) allow solar radiations to enter but prevent passage of heat waves to the outside.
Green House Gases (GHGs). CO2, methane (CH4).
Question 40.
What are the two forms of oxygen found in the atmo¬sphere ? Write one importance of each. (CCE 2013)
Answer:
Diatomic oxygen (O2) and triatomic ozone (O3). Importance,
- Oxygen is essential for respiration and combustion,
- Ozone present in stratosphere protects earth from harmful short wave ultraviolet radiations (100-320 nm).
Question 41.
Draw carbon cycle in nature. (CCE 2013)
Answer:
Question 42.
Your school is less than a kilometre from your house. Your neighbourhood friend who attends the same school, comes to the school by a car every day.
How do you think he can contribute towards reduction of air pollution ? Write any three suggestions. (CCE 2013)
Answer:
- My friend can join a car pool for school.
- He can go to school in school bus.
- Since the distance is small, my friend can go to school by foot or on bicycle.
Question 43.
What are CFCs ? Why are they harmful ? (CCE 2013)
Answer:
CFCs are chlorofluorocarbons that have been in use as aerosol propellents, refrigerants, shaving foams, formation of foam sheets and mattresses, spray agents, etc. They pass into stratosphere and release chlorine. Chlorine destroys ozone molecules and convert them into oxygen. As a result ozone present in stratosphere gets depleted resulting in passage of harmful UV radiations on earth.
Question 44.
List any three human activities that you think would lead to increase in carbon dioxide content of atmosphere.
(CCE 2013)
Answer:
- Large scale deforestation that reduces assimilation of CO2 in photosynthesis,
- Combustion of fossil fuels in thermal plants, industries, transport etc. is throwing large amounts of CO2 into the atmosphere.
- Biomass or wood burning.
Raj at and five of his friends went for a picnic with a lot of food stuffs and game items to Gorewara lake from where the drinking water supply for the city is done. As they were playing, they saw a fish entangled in the twigs and injured itself. They helped to free it, nurtured it and put it back in water. Before leaving, they put all the left-over food in water.
Question 45.
Answer the following questions.
- What would have happened had they not saved the fish ?
- What do you think about their act of throwing the food into water ?
- Mention one way in which you will help your city authorities to cope with such problem. (CCE 2013)
Answer:
- The fish would have died. Saving and nurturing an animal is an act of love for nature.
- The act of throwing the left-over food in the lake is bad as it increases the organic loading of lake and can cause its eutrophication.
- To install two types of bins on the shore of the lake for placing left-overs by the visitors.
Question 46.
In coastal areas what is the direction of flow of air during the day ? Explain why ? (C. C.E. 2014)
Answer:
Sea Breeze: It is flow of cooler breeze from sea towards the land during the day time. During day time, land gets heated faster. Air above land heats up, rises and creates an area of low pressure. Air above sea is cooler and has higher pressure. It moves towards land.
Question 47.
What is the percentage of oxygen found in the atmosphere ?
Name two compounds of oxygen found in nature. Name any three processes in which oxygen is used up in atmosphere. (C.C.E. 2014)
Answer:
21% (20-94%). Diatomic O2(oxygen) and triatomic O3(ozone).
Uses:
- Respiration in most organisms,
- Combustion of fossil fuels,
- Formation of oxides,
e.g., nitrogen oxides during atmospheric fixation of nitrogen, - Decomposition of organic matter.
Question 48.
State three factors influencing wind movements which re-sult in diverse atmospheric phenomena. (C.C.E. 2014)
Answer:
Winds are basically caused by heating of air in certain parts. The hot air rises upwards. This creates an area of low pressure. Cooler air from adjacent higher pressure areas passes into this area. This creates wind. The factors which control movement of winds in different directions in different parts of the earth are
- Uneven heating of land in different parts of earth
- Differences in heating and cooling of land and water.
- Barrier of mountains
- Rotation of earth.
Question 49.
Explain the biological processes in nitrogen cycle.
(C.C.E. 2014)
Answer:
Biological processes in nitrogen cycle are :
- Biological Nitrogen Fixation: A number of free living and symbiotic bacteria and blue-green algae are capable of picking up of atmospheric nitrogen and converting it into ammonia that combines with organic acids to form amino acids. Rhizobium is the common symbiotic bacterium that occurs in the nodules of legume roots.
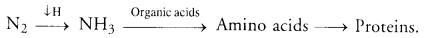
- Nitrogen Assimilation: It is carried out by plants. Plants pick up nitrates or ammonium ions from their medium. Nitrate or nitrite is first changed into ammonium state. Ammonium ions combine with organic acids to form amino acids. Amino acids give rise to proteins and nucleotides. Nucleotides produce nucleic acids. Enzymes are derived from proteins.
- Decomposition: Organic remains undergo decay and decomposition through the agency of decomposers. It releases carbon dioxide.
- Denitrification: It is the process of reduction of nitrates into gaseous nitrogen which escape into atmosphere. Denitrification is caused by bacteria present in water logged soils, g., Pseudomonas aeruginosa.
Question 50.
How is percentage of carbon dioxide maintained in the atmosphere ? Mention one human activity which adds carbon dioxide in the atmosphere. (C. C.E. 2014)
Answer:
Percentage of carbon dioxide is maintained in the atmosphere by its consumption in photosynthesis (carbon assimilation) being almost equal to its liberation in respiration and combustion.
However, with increasing use of fossil fuels, more carbon dioxide is being added to the tmosphere than its withdrawal.
Question 51.
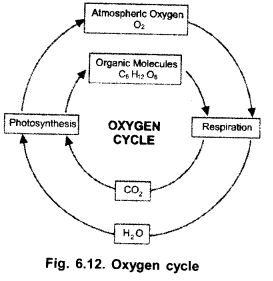
Draw a labelled diagram of‘oxygen cycle’ occurring in nature. (C.C.E. 2014)
Answer:
Question 52.
Write the composition of soil. On what basis is the type of soil decided ? (C. C.E. 2014)
Answer:
Soil Composition: Soil consists of mineral matter (45%), organic matter (5%), air (25%), water (25%) and variable number of soil organism. Mineral matter consists of gravel (size 2-10 mm), sand (0.02-2.0 mm), silt (0.002-0.02 mm) and clay (below 0.002 mm) Organic matter is in semidecayed form and is called humus.
Type of Soil: Average particle size determines the structure j of soil, e.g., clayey, loam, sandy. Soil particles determine j mineral availability, soil hydration and soil aeration.
Question 53.
(a) What is the outermost layer of the earth called ?
(b) How is it important to life forms ?
(c) Name the factors that help in the formation of soil from rocks. (C. C.E. 2014)
Answer:
Answer:
(a) Lithosphere.
(b) Upper crust of earth forms soil which provides anchor¬age, water and minerals, to terrestrial plants. Aquatic organ¬isms also depend upon soil for supply of minerals.
(c) Soil is formed through two processes of weathering and humification.
Weathering
It is pulverisation of rocks or breaking of rocks into fine particles. There are three types of weathering — physical (atmospheric changes and mechanical forces), chemical and biological. Sun, water, wind and living organisms perform them.
- Sun: It causes expansion of rocks by heating. Cooling causes their contraction. Different parts of rocks expand and contract at different rates. Uneven expansion and contraction produces cracks leading to fragmentation of rocks.
- Water:
- Wetting and Drying: Certain rock components can pick up and lose moisture. They undergo swelling and contraction resulting in fragmentation of rocks.
- Frost Action: Water seeping in cracks would swell up and exert a great pressure if it freezes due to low temperature. The rock would undergo fragmentation.
- Abrasion: Running water carrying rock fragments would break and grind rocks occurring in the pathway. Rain and hail also cause rock breaking.
- Wind: Dust and fine sand carried by wind cause abrasion of the rock surface when wind strikes the same.
- Living Organisms: Lichens secrete chemicals to dissolve minerals from the rock surface. This produces crevices where dust collects. Mosses grow there. They cause deepening of crevices and development of small cracks. Roots of short lived plants widen these cracks. Roots of larger plants cause fragmentation of rocks by entering the cracks and growing in size.
Humification
Partially decomposed organic matter or humus mixes with weathered rock particles to form soil. Humus helps in formation of soil crumbs which are essential for maintaining proper hydration and aeration of soil.
Question 54.
(a) State the reason for the following :
(t) Excess burning of coal causes green house effect,
(it) Soil is a mixture.
(iii) Temperature ranges from -190° C to 110°C on the surface of the moon. (C.C.E. 2014)
Answer:
(a) Excess burning of coal adds lot of CO2 to the atmosphere. Carbon dioxide is a green house gas which allows the solar radiations to strike the earth’s surface but prevents the escape of long wave radiations from the earth’s atmosphere.
(b) Soil is a Mixture. Soil consists of particles (sand, silt, clay and humus) which do not get dissolved in water, neither they react with one another.
(c) Moon does not have an atmosphere. Its surface heats up to 110°C when solar radiations strike it. In the absence of solar radiations, the surface temperature of moon dips to -190°C.
Question 55.
State the different ways by which atmospheric nitrogen can be fixed into oxides of nitrogen. Explain in brief.
(C.C.E. 2014)
Answer:
Atmospheric Nitrogen Fixation: In the presence of lightning (energy and pressure), electric storm and high energy ultraviolet rays, small amounts of nitrogen and oxygen of the air react to form nitrogen oxides. The latter dissolve in rain water to form nitric and nitrous acids. They pass down to soil and water bodies forming nitrites and nitrates.
- Industrial Nitrogen Fixation: Nitrogen is made to combine with hydrogen under high temperature and pressure to form ammonia. Ammonia is often changed further to urea because the latter is less toxic.
- Nitrogen Assimilation: It is carried out by plants. Plants pick up nitrates or ammonium ions from their medium. Nitrate or nitrite is first changed into ammonium state. Ammonium ions combine with organic acids to form amino acids. Amino acids give rise to proteins and nucleotides. Nucleotides produce nucleic acids. Enzymes are derived from proteins.
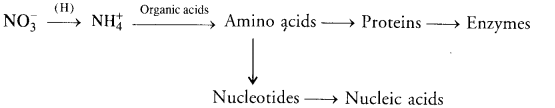
- Decomposition: Organic remains undergo decay and decomposition through the agency of decomposers. It releases carbon dioxide.
- Denitrification: It is the process of reduction of nitrates into gaseous nitrogen which escape into atmosphere. Denitrification is caused by bacteria present in water logged soils, g., Pseudomonas aeruginosa.
Question 56.
Describe how lichens and big trees influence the formation of soil.
Answer:
Living Organisms: Lichens secrete chemicals to dissolve minerals from the rock surface. This produces crevices where dust collects. Mosses grow there. They cause deepening of crevices and development of small cracks. Roots of short lived plants widen these cracks. Roots of larger plants cause fragmentation of rocks by entering the cracks and growing in size.
Question 57.
Mention six combined forms in which oxygen is found on the earth’s crust. (C. C.E. 2014)
Answer:
Carbonate, silicon oxides, sulphate, nitrate, phosphate, metal oxides.
Question 58.
Explain the following terms :
(i) Nitrogen Fixation
(ii) Nitrification
(iii) Denitrification. (C. C.E. 2014)
Answer:
(i) Nitrogen Fixation: It is conversion of inert gas into bio¬logically acceptable form. Nitrogen fixation is carried out abiologically in the atmosphere (through lightning, electric storms, UV radiations) and biologically by certain bacteria and cyanobacteria (= blue-green algae).
(ii) Nitrification : Conversion of ammonia to nitrites and nitrites to nitrates by bacteria.
(iii) Denitrification: Conversion of nitrates to gaseous nitrogen by bacteria.
Long Answer Questions (5 Marks)
Question 1.
(i) Make neat and labelled sketch of nitrogen cycle in nature.
(ii) Describe in brief the role of nitrogen fixing bacteria and lightning in fixing nitrogen. (CCE 2011, 2014)
Answer:
(i)
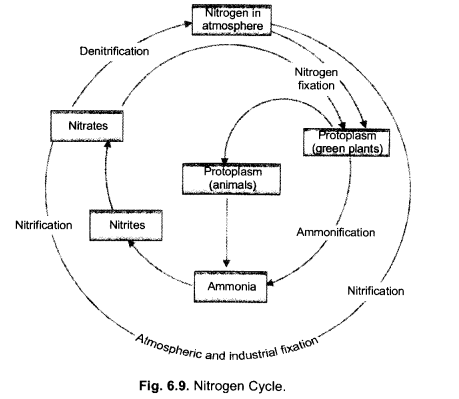
(ii)
(a) Nitrogen Fixing Bacteria (Biological Nitrogen Fixation). They live both freely (e.g., Azotobacter) as well as symbionts (e.g., Rhizobium in root nodules of legumes). The bacteria pick up nitrogen gas (N2) from soil atmosphere, reduce it to ammonia (NH3), then forming amino acids and proteins.
(b) Lightning (Physical Nitrogen Fixation). By its energy, it helps to combine a small quantity of nitrogen with oxygen of the atmosphere forming nitrogen oxides. The latter dissolve in rain water and enter the soil producing nitrite and nitrate.
Question 2.
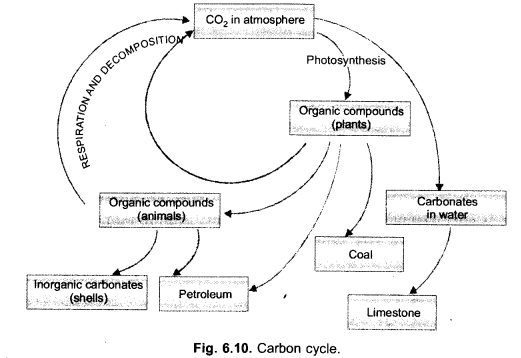
(i) Draw a neat labelled sketch of carbon cycle in nature.
(ii) What is green house effect ? How does CO2 cause global warming in the atmosphere ?
(CCE 2011, 2012, 2013)
Answer:
(i)
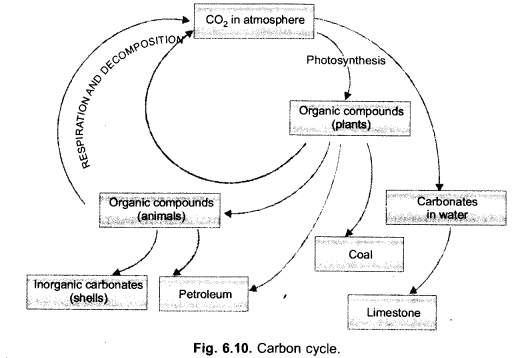
(ii) Green house effect is keeping an area warm by allowing the solar radiations to pass in but preventing long waves to escape due to presence of radiatively active gases and glass panes.
CO2 is a green house gas. It permits the solar radiations to pass through and reach the surface of the earth. Infra-red (heat) waves emitted by the earth are, however, not allowed to pass out by CO2. They are reflected back to earth. This causes global warming.
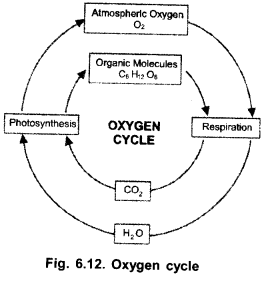
Question 3.
(a) Mention the two forms of oxygen found in the atmosphere,
(b) Name the form of oxygen, absorbing UV radiations.
(c) Draw flow diagram of oxygen cycle. (CCE 2011)
Answer:
(a) O2, O3
(b) O3
(c)
Question 4.
Many human activities lead to increasing levels of pollution of air, water bodies and soil. “Isolating these activities to specific and limited areas would not help in reducing pollution.” Justify the statement giving at least five reasons. (CCE 2011, 2012)
Answer:
It is often suggested that all polluting units be asked to shift outside the city limits, so that there is not much effect on the residential area. This is, however, of not much help as
- Burning of fossil fuels lead to acid rain which spreads over large areas killing vegetation, water bodies and causing harm to human beings and their assets.
- Polluting units add green house gases into the atmosphere. They have global warming effect.
- A number of air pollutants pass into stratosphere causing depletion of protective ozone layer.
- Passage of sewage into water bodies makes the water unfit for consumption as it becomes a source of many water borne diseases. There is scum, foul odour and bad taste.
- Dumping of industrial effluents over the soil causes many of the toxic chemicals and heavy metals to slowly percolate down and get mixed with ground water.
Question 5.
(a) Mention three processes in which oxygen is used up from the atmosphere and the only process in which it is returned to the atmosphere.
(b) Describe briefly two processes by which carbon dioxide is returned back to the atmosphere.
(CCE 2011, 2012)
Answer:
(a)
- Consumption of Oxygen.
- Respiration
- Combustion
- Atmospheric fixation of nitrogen.
- Return of Oxygen. Photosynthesis,
(b) Return of CO2.
- Respiration
- Combustion.
Question 6.
(a) With the help of a well sketched diagram, explain water cycle in nature.
(b) How is green house effect related to global warming ? Explain. (CCE 2011, 2012)
Answer:
(a)
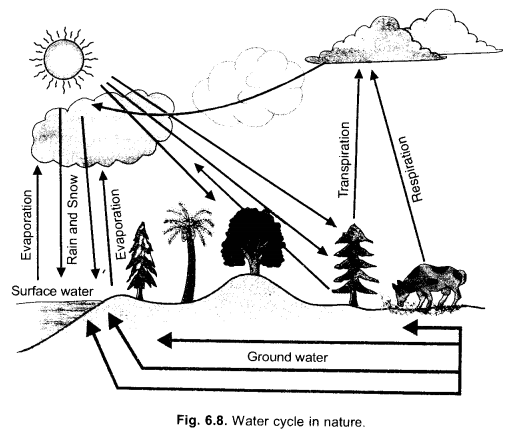
Water evaporates from the wet surfaces and plants (transpiration). The water vapours being light rise in the air, getting cooled and condensed over dust particles forming clouds. Clouds move along air currents, , cooling further and undergoing precipitation.
(b) Green house effect is keeping an area warm by allowing the solar radiations to pass in but preventing long waves to escape due to presence of radiatively active gases and glass panes.
Green house effect is shown by a number of radiately active gases, also called green house gases (GHGs), e.g., CO2, methane, chlorofluorocarbons, nitrous oxide. The gases allow the solar radiations to pass through and reach the surface of earth. However, heat waves (infra-red rays) coming from hot surfaces of earth are unable to pass out of the atmosphere as the higher concentration of green house gases reflect most of them back to earth. This raises the temperature of the earth. The phenomenon is called global warming.
Question 7.
(a) In coastal area, wind current moves from sea towards the land during the day but during night it moves from land to sea. Discuss the reason.
(b) How are CFCs harmful for the environment and living beings ? (CCE 2011, 2012)
Answer:
(a)
- During day, warmer land heats up the air above it which rises and creates an area of low pressure. Air above sea remains comparatively cool. It has a higher pressure and, therefore, cooler air moves towards the land,
- During night, land becomes cooler quickly while sea water remains comparatively warmer. Low air pressure area develops over sea. Cooler air from land, will therefore, flow towards sea.
(b) CFCs passing into stratosphere split up to release active chlorine under the impact of UV radiations. Chlorine catalyses the breakdown of ozone into oxygen. Depletion of ozone will allow more UV rays to pass down to earth causing skin cancers, blindness and weakening of immune system.
Question 8.
(a) What do you understand by ozone layer depletion ?
(b) What is air pollution ? How does air pollution affect animal and plant life ? (CCE 2011)
Answer:
(a) Ozone Layer Depletion: Ozone hole is formed during spring time over antarctica and to a small extent over north pole. Thinning of ozone layer or ozone hole increases the passage of harmful ultravoilet rays to earth. This has increased incidence of skin cancers, defective eye sight, reduced immunity, increased number of mutations and reduced crop yield in southern countries of southern hemisphere.
(b) Air Pollution: It is the addition of substances, gases and chemicals into the air that has the harmful effect on human beings, human assets, plants and animals. The major sources of air pollution are combustion of fossil fuels, industrial processes and agricultural burning. Air pollutants include suspended particulate matter (SPM), gases (e.g., sulphur dioxide, nitrogen oxides, carbon monoxide, hydrogen sulphide, methane, ammonia) and vapours of unburnt hydrocarbons. They also produce two harmful environmental products of acid rain and smog.
Effect on Animal Life. Respiratory disorders, allergies, eye and throat irritation, haemorrhages, frequent diarrhoea, reduced appetite and weakening of bones.
Effect on Plant Life. Chlorosis, necrosis, water soaked areas, defoliation and die back.
Question 9.
How do clouds form in the sky ? Draw the biogeochemical cycle involved in it. What are the different states in which water is found in the water cycle ?
(CCE 2011)
Answer:
(a) Clouds are wet air masses that float in the direction of prevailing wind. They develop when water vapours are formed in large number. There is evaporation from the surface of water bodies and wet areas due to their heating during the daytime. Plants also give out water vapours in transpiration while animals do so in exhaled air and perspiration. Air also heats up during daytime. The hot air along with water vapour rises up. At a height, air expands and becomes cool. Cooling causes the water vapours to condense. Suspended particles of dust and other materials function as nuclei around which water vapours condense. When a large wet air mass collects, cloud is formed.
(b)
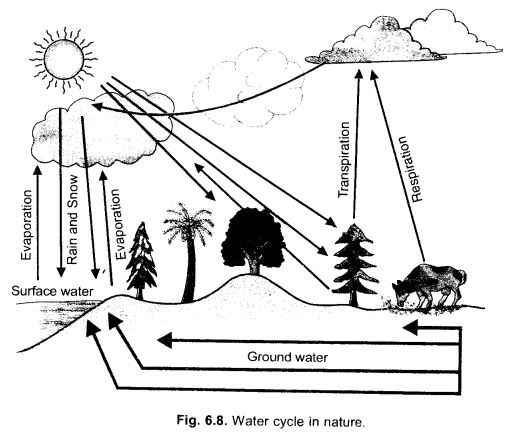
(c) States of Water,
- Gaseous state – as vapour,
- Liquid state – as rain, flowing water, water bodies, underground or soil water.
- Solid state – as snow in cooler areas like polar regions, mountain tops, winter in subarctic and temperate areas.
Question 10.
(a) Draw a labelled diagram to show oxygen cycle in biosphere,
(b) Name the major process through which oxygen is returned to the atmosphere
(c) Enlist the various processes by which atmospheric oxygen and oxygen dissolved in water is consumed.
(CCE 2011)
Answer:
(a)
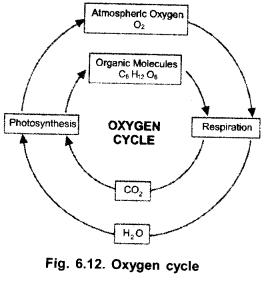
(b) Photosynthesis,
(c)
- Respiration (consumes both atmospheric oxygen and oxygen dissolved in water),
- Combustion.
- Decomposition.
Question 11.
(a) Describe briefly any two processes involved in the cycling of N2 in the environment.
(b) What is nitrogen fixation ? Why do plants need to fix nitrogen ? (CCE 2011)
Answer:
(a) Processes Involved in Cycling of N2 :
- Nitrogen fixation,
- Nitrogen assimilation
- Decomposition
- Denitrification.
(b) Nitrogen Fixation: It is conversion of inert nitrogen gas (N2) into biologically acceptable forms like NH3, NO2, NO3. Plants need nitrogen fixation because they cannot absorb and utilise nitrogen gas.
Question 12.
(a) What are biogeochemical cycles ?
(b) Why is circulation of water necessary in the environment ? Discuss any two human activities which are disturbing water cycle.
(CCE 2011)
Answer:
(a) The repeated circulation of biogeochemicals or biogenetic nutrients between abiotic and biotic components of the environment is called biogeochemical cycling, e.g., CO2 cycle, N2 cycle, Water cycle, O2 cycle.
(b)
- Circulation of water is necessary because land biota needs fresh water while the major reservoir of water is ocean. Land is regularly losing water through evaporation, flowing of rivers and ground water to the oceans. Therefore, land must receive fresh water regularly in the form of rain and snow.
- Excessive withdrawal of ground water.
- Polluting water bodies through sewage, industrial effluents, fertilizers and pesticides.
Question 13.
(a) Describe the role of photosynthesis and respiration in carbon cycle.
(b) What is meant by biogeochemical cycle ? Name two essentials which are transferred between different components of the biosphere. (CCE 2011)
Answer:
(a) (i) Photosynthesis. It assimilates CO2 and forms organic food like glucose and starch, proteins and fats. Organic food with its contained energy is passed from producers to herbivores and then to carnivores in the ecosystem.
(ii) Respiration. It oxidises glucose and other organic compounds-for releasing energy for body activities. CO2 is liberated.
(b) Biogeochemical Cycle: The repeated circulation of biogeochemicals or biogenetic nutrients between abiotic and biotic components of the environment is called biogeochemical cycling, e.g., CO2 cycle, N2 cycle, Water cycle, O2 cycle.
Biogeochemicals and Energy: Biogeochemicals are picked up by plants and made part of organic matter which also incorporates chemical energy during the process of photosynthesis. From plants, the biogeochemicals and energy contained in organic food pass into animals which feed on plants. Animals are eaten by other animals. The biogeochemicals and energy contained in organic matter pass into them. However, the whole of energy is not passed on the higher trophic levels. Energy is dissipated at every step of its transfer. Organic wastes and dead bodies of all organisms are acted upon by decomposers to release biogeochemicals back into abiotic environment.
Question 14.
(a) Fill in A, B, C, D and E
(b) What will happen if step A does not occur ?
(c) What is the role of nitrogen fixing bacteria in the biosphere ?
(d) Name two biologically important compounds that contain both oxygen and nitrogen. (CCE 2011)
Answer:
(a)
A – ammonification.
B – ammonia.
C – nitrites.
D – nitrates.
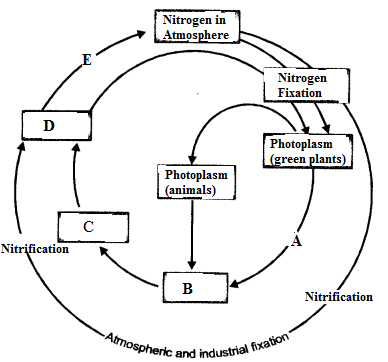
(b) Absence of Step A. No breakdown of proteins to release ammonia.
(c) Role of Nitrogen Fixing Bacteria. They add a lot of usable form of nitrogen in the soil which become available to plants for synthesis of organic matter.
(d) Nucleic acids (DNA and RNA) and proteins.
Question 15.
(a) What do the blanks 1, 2, 3, 4 and 5 in the given cycle stand for ?
(b) Name two natural and one man-made process by which CO2 returns to the atmosphere
(c) CO2 is necessary for plants but it is also a pollutant. Justify your answer.
(CCE 2011)
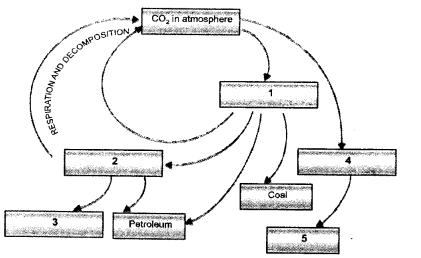
Answer:
(a)
1. – organic compounds (plants).
2. – Organic compounds (animals)
3. Inorganic carbonates (shells)
4. – carbonates in water.
5. – Limestone,
(b) Natural Processes of Return of CO2.
- Respiration,
- Decay and decomposition. Man-made Process of Return of C02. Combustion
(c) Besides being essential for photosynthesis, carbon dioxide is a green house gas. Upto 350 ppm, it is both a good raw material as well as essential for keeping the earth warm. However, when its concentration rises (as presently it is 400 ppm), it becomes pollutant because it results in global warming. The latter is quite dangerous because it will melt polar ice, raise the water level of oceans and submerge several coastal areas and islands.
Question 16.
(a) Why step farming is common in hills ?
(b) How is acid rain causing harm to ‘Taj Mahal’
(c) Name two leguminous plants which can fix nitrogen. (CCE 2011)
Answer:
(a) Step Farming in Hills. In hills, slope is divided into a number of flat fields. The process is called terracing or step farming. It helps in slowing down the flow of water, increasing water absorption by soil and decreasing soil erosion.
(b) Acid rain contains small quantities of sulphuric acid and nitric acid. They have a corrosive action on marble
(calcium carbonate) forming calcium sulphate and calcium nitrate that peels off. Taj Mahal being made of marble with specific engravings will lose its charm due to acid rain corrosion.
(c) Gram, Pea, Groundnut.
Question 17.
(a) How does energy enter the biosphere ?
(b) In the following biogeochemical cycle, name and define the process marked as (X), (Y) and (Z).
(c) What compounds of nitrogen cause air pollution and how are they released in air ? (CCE 2011)
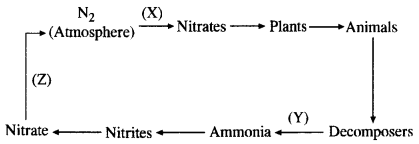
Answer:
(a) Energy enetering the biosphere comes from sunlight. It is picked up by green plants (with the help of chlorophyll) and changed into chemical energy during photosynthesis when organic matter is built up.
6CO2 + 6H2O + Energy ————> C6H12O6 + 6O2
(b) (X) – Nitrogen Fixation.
(Y) – Ammonification.
(Z) – Denitrification.
(c) Oxides of nitrogen cause air pollution. Most of them are released during combustion of fossil fuels.
Question 18.
(a) The circulation of carbon is important in nature. Give reasons for your answer,
(b) Explain any two processes involved in the cycling of nitrogen in the environment.
(CCE 2011, 2012)
Answer:
(a) Carbon is an essential component of all organic substances. However, its concentration in the atmosphere is very small. Therefore, circulation of carbon is important for the continuation of life on earth. Organic substances are formed from CO2 during the process of photosynthesis. Organic matter passes from plants to animals, animals to animals and ultimately to decomposers. It is regularly consumed in respiration by all living beings which releases CO2. CO2 is also released during decomposition of organic matter.
(b)
- Nitrogen fixation,
- Nitrogen assimilation
- Decomposition involving ammonification and nitrification,
- Denitrification.
Question 19.
(a) Explain two major processes that maintain CO2 percentage in the atmosphere,
(b) Draw carbon cycle.
(c) Mention two steps involved in carbon cycle.
(CCE 2011)
Answer:
(a) The two main process that maintain CO2 percentage in the atmosphere are photosynthesis (withdrawal of CO2) and respiration (release of CO2).
(b)
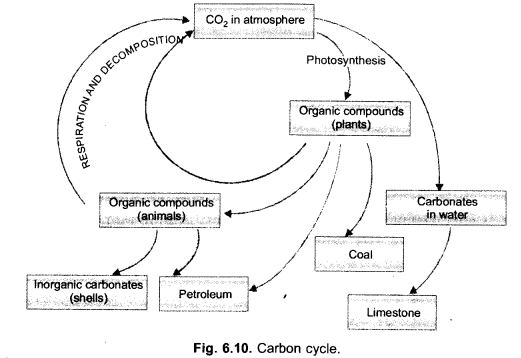
(c) Steps,
- Photosynthesis. It withdraws CO2 from environment and incorporates carbon into organic food along with energy.
- Passage. Organic food synthesised by plants becomes available to herbivores. From herbivores, it passes to carnivores.
- Respiration. Organic food not only builds up the body of organisms but also becomes substrate for respiration wherein it releases CO2 back into environment.
- Combustion. It is burning of organic matter including fossil fuels. Combustion releases CO2.
- Decay and Decomposition. Organic remains are acted upon by decomposers. The process releases CO2.
- Long Term Withdrawal and Return. Seepage of CO2 rich water forms carbonate rocks like limestone. Carbonate also gets incorporated inside shells and skeleton of animals.
Large scale burying of organic life inside earth produces fossil fuels. Weathering of carbonate rocks and burning of fossil fuel release locked CO2 back to environment.
Question 20.
(a) Name any four carbon containing molecules which are essential to life forms.
(b) Mention the three processes in which oxygen is used up from the atmosphere and the only process in which it is returned to the atmosphere. (CCE 2011, 2012)
Answer:
(a) Proteins, Fats, Carbohydrates, Nucleic acids (DNA, RNA).
(b) Use Up of Oxygen. Respiration, Combustion, Atmospheric fixation of nitrogen, Return of oxygen. Photosynthesis.
Question 21.
Explain nitrogen cycle with the help of labelled diagram.
Name four processes which occur during nitrogen cycle.
(CCE 2012)
Answer:
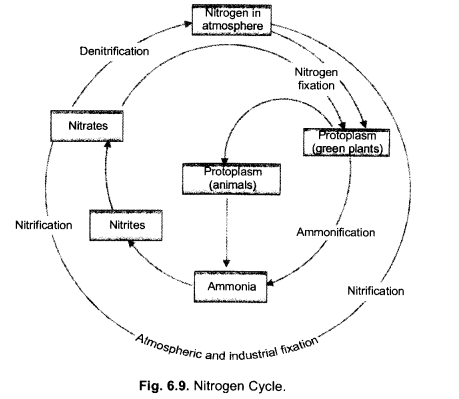
Nitrogen occurs as inert gas (N2) in atmosphere (78% of total). A part of it is converted into useful compounds of nitrogen by atmospheric fixation, biological fixation and industrial fixation. The same are absorbed by plants and assimilated to form protoplasmic substances. Animals obtain the same from plants. Organic remains are acted upon by decomposers to release ammonia from which nitrite and nitrate are formed through activity of other groups of microbes. The same are picked up by plants again. A part of nitrate present in water-logged soils is changed to gaseous nitrogen by denitrifying bacteria.
Processes:
- Nitrogen fixation.
- Nitrogen assimilation.
- Decomposition.
- Denitrification.
Question 22.
(a) Name the gas which is used in the process of photosynthesis.
(b) Complete the cycle (as given in question 15) and explain. (CCE 2012)
Answer:
(a) CO2.
(b)
1 – organic compounds (plants).
2 – Organic compounds (animals)
3- Inorganic carbonates (shells)
4 – carbonates in water.
5 – Limestone,
CO2 present in atmosphere is picked up by photoautotrophs in their photosynthesis.
They form organic substances. Animals obtain the same from plants. Respiration of living beings and decomposition of organic matter return CO2 back to atmosphere. A part of carbon passes , into earth as carbonate forming limestone.
Some animals also form shells from their carbonaceous materials. Both the processes take out a part of CO2 from the cyclic pool.
Question 23.
(a) Draw a schematic diagram of nitrogen cycle in nature.
(b) Explain how the nitrogen molecules are converted into nitrates and nitrites by
- Biological process,
- Physical process.
(CCE 2012, 2014)
Answer:
(a)
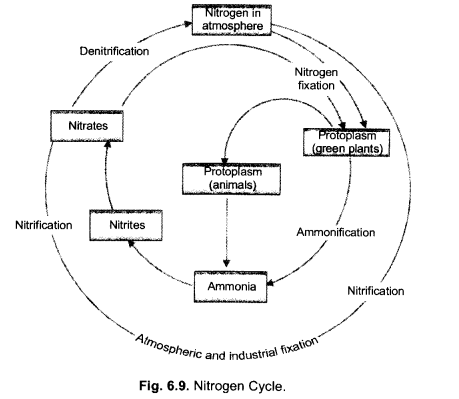
(b)
- Biological Process. N2 is fixed biologically by some free living (e.g, Azotobacter) and symbiotic (e.g., Rhizobium) prokaryotes to produce ammonia which is immediately fixed into amino acids from which proteins, nucleotides and other compounds are formed. After death of the organism, the proteins are decomposed to release ammonia (ammonification). Ammonia is oxidised to nitrite {e.g., Nitrosococcus) and then nitrate (e.g., Nitrobacter) in the process of nitrification.
- Physical Process. Ligthing, electric storm and high energy ultra-violet rays help combine small quantity of nitrogen with oxygen to form nitrogen oxides. The latter dissolve in rain water to produce nitrite and nitrate.
Question 24.
(a) Write any two uses of carbon dioxide gas.
(b) List the importance of oxygen gas and ozone gas present in the atmosphere.
(c) How atmosphere of earth differ from that of Mars and Venus ?
(d) Mention the percentage of earth covered by H2O.
(CCE 2012)
Answer:
(a) Uses of CO2.
- It is raw material for photosynthesis.
- Being a major green house gas, it keeps the earth comfortably warm for living beings.
(b)
- Importance of Oxygen. It is essential for respiration and combustion.
- Importance of Ozone. Ozone is harmful in trophosphere where it is unstable. It is useful in stratosphere where it forms a shield to protect the earth against very harmful high energy UV radiations.
(c)
| Atmosphere over Earth | Atmosphere over Venus and Mars | ||
| 1. | Nitrogen and Oxygen. | It contains nitrogen and oxygen. | The two are absent |
| 2. | Carbon Dioxide. | Carbon dioxide content is little (0-03%). | Carbon dioxide is the major component of atmosphere forming 95-97%. |
| 3. | Water Vapours. | The atmosphere contains water vapours which form a component of water cycle. | The atmosphere does not contain water vapours. Living beings, being absent, have no role in |
| 4. | living Beings. | Composition of atmosphere is maintained by living beings. | determining composition of atmosphere. |
(d) 75%.
Question 25.
(a) Increased loading of nutrients in the water body results in algal bloom. How is it harmful to aquatic life ?
(b) What is acid rain ? Name the two gases which causes acid rain.
(c) List any two harmful effects of air pollution.
(CCE 2012)
Answer:
(a) Increased loading of nutrients in the water body results in eutrophication stimulating the growth of photoautorophic organisms, especially algae. The algae grow in such abundance as to cover the whole water surface. The phenomenon is called algal bloom. It shades the submerged plants which die. Decomposers become active but pick up most of the oxygen dissolved in water. Depletion of oxygen kills the animals, further increasing organic loading. Ultimately, anaerobic breakdown of organic matter begins which produces sulphides that form scum and sludge in the water body. Blue-green algae often invade such waters and release toxins.
(b) Acid rain is rainfall having a pH of less than 5. It is caused by dissolution of two gases, SO2 and nitrogen oxides, in rain water.
(c) Harmful Effects of Air Pollution,
- Suspended particulate matter or SPM causes asthma, bronchitis and allergic cold.
- Pollutant gases cause irritation in eyes, throat and lungs, damage liver, kidneys, spleen and nervous system.
- Hydrocarbons and some gases cause cancer.
Question 26.
(a) Name any four cycles in nature that form part of biogeochemical cycling.
(b) Name any four sources of fresh water.
(c) How does energy enter biosphere ?
(d) Name one natural and one man made process by which carbon dioxide returns to atmosphere.
Answer:
(a) Water cycle, nitrogen cycle, carbon cycle, oxygen cycle.
(b) Sources of Fresh Water,
- GroundWater: It is brought to the surface through water pumps, dug wells, tube- wells, etc.
- Water Reservoirs: They store run off and rain water.
- Rain: It brings fresh water every where that can be stored.
- Rivers: They develop through melting of snow and formation of springs in the catchment areas. Rivers supply fresh water all through their passage to sea.
(c) Energy enters the biosphere through trapping of solar energy by photcautotrophs during the process of photosynthesis.
(d)
- Natural Process Respiration
- Man-Made Process. Combustion.
Question 27.
(a) How is gaseous nitrogen fixed by the plants ?
(b) Mention one difference between nitrogen fixation and nitrification.
(c) Mention the percentage of oxygen content in the atmosphere.
(d) Name three combined forms of oxygen in which it is found. (CCE 2012)
Answer:
(a) Plants can fix gaseous nitrogen only with the help of certain bacteria and blue-green algae, e.g, Rhizobium inside root nodules of legumes.
(b) Nitrogen fixation is conversion of inert gaseous nitrogen into biologically usable salts of nitrogen (e.g., nitrite, nitrate, ammonia) by physical and biological methods. Nitrification is conversion of ammonia (released during decomposition of proteins) into nitrates with the help of certain bacteria (e.g., Nitrosococcus, Nitrobacter).
(c) 21%
(d) Carbon dioxide, sulphur dioxide, nitrogen oxides.
Question 28.
(a) Explain the biological and physical methods of nitrogen fixation.
(b) (i) Complete the nitrogen cycle by labelling ‘X’ and ‘Y in the biogeochemical cycle shown.
(ii) Explain the part marked ‘X’. (CCE 2012)
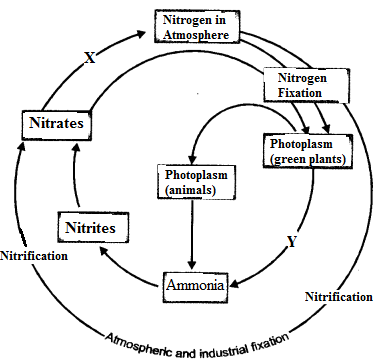
Answer:
(a) Nitrogen Fixation: It is conversion of inert gaseous nitrogen into usable form.
- Biological Nitrogen Fixation: It is carried out by free living (e.g., Azotobacter) and symbiotic (e.g., Rhizobium) bacteria. They pick up free nitrogen gas from soil air and convert it into ammonia state. The latter is used to change organic acids into amino acids.
- Physical Nitrogen Fixation: (Atmospheric Nitrogen Fixation). In the presence of lightning, electric storm and high energy UV rays, small amounts of nitrogen and oxygen combine to form nitrogen oxides. They dissolve in rain water and pass down to earth as nitrites and nitrates.
(b) Y-Ammonification.
X-Denitrification
(ii) Denitrification: Some bacteria present in water-logged soils take oxygen from nitrates for their activity. This changes nitrates into gaseous nitrogen.
Question 29.
(a) Observe the diagram. When the incense stick is kept near the edge of the mouth of beaker, in which direction will the smoke flow ? Give reason.
(b) What role does the sun play in formation of soil ?
(CCE 2012)
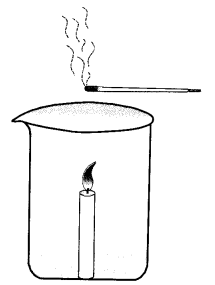
Answer:
(a) The incense will flow towards the flame because it is creating an area of low air pressure due to hot air rising upwards.
(b) Soil is formed through two processes of weathering and humification.
Weathering
It is pulverisation of rocks or breaking of rocks into fine particles. There are three types of weathering — physical (atmospheric changes and mechanical forces), chemical and biological. Sun, water, wind and living organisms perform them.
- Sun: It causes expansion of rocks by heating. Cooling causes their contraction. Different parts of rocks expand and contract at different rates. Uneven expansion and contraction produces cracks leading to fragmentation of rocks.
- Water:
- Wetting and Drying: Certain rock components can pick up and lose moisture. They undergo swelling and contraction resulting in fragmentation of rocks.
- Frost Action: Water seeping in cracks would swell up and exert a great pressure if it freezes due to low temperature. The rock would undergo fragmentation.
- Abrasion: Running water carrying rock fragments would break and grind rocks occurring in the pathway. Rain and hail also cause rock breaking.
- Wind: Dust and fine sand carried by wind cause abrasion of the rock surface when wind strikes the same.
- Living Organisms: Lichens secrete chemicals to dissolve minerals from the rock surface. This produces crevices where dust collects. Mosses grow there. They cause deepening of crevices and development of small cracks. Roots of short lived plants widen these cracks. Roots of larger plants cause fragmentation of rocks by entering the cracks and growing in size.
Humification
Partially decomposed organic matter or humus mixes with weathered rock particles to form soil. Humus helps in formation of soil crumbs which are essential for maintaining proper hydration and aeration of soil.
Question 30.
(a) Complete the given oxygen cycle.
(b) State four forms in which oxygen is found in the earth’s crust.
(c) Give an example where oxygen does not support life activities. (CCE 2012)
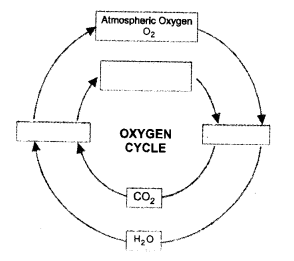
Answer:
(a) Organic molecules, Respiration, Photosynthesis.
(b) Carbonate, sulphate, nitrate, metal and silicon oxides.
(c) Anaerobic organisms and deep water forms, e.g, Clostridium botulinum.
Question 31.
“Urbanisation and industrialisation are mainly responsible for increase in environment pollution”. Justify this statement and suggest ways and means to check it. (CCE 2012)
Answer:
Urbanisation and industrialisation generate a lot of pollutants:
- Sewage: A lot of sewage is generated in urban areas. It contains organic matter and several pathogens.
- Industrial Effluents: They are produced by most industries like dyeing units, electroplating units, paper mills, leather processing units, sugar mills, chemical industries, etc. Like sewage , the industrial effluents are disposed off by passing into a water body.
- Fossil Fuels: Homes, industries, automobiles and thermal plants are burning fossil fuels that produce SPM, pullutant gases and hydrocarbon vapours.
- Processing Industries: They generate pollutant particulate matter like cotton dust, coal dust, iron dust, asbestos, etc.
- Garbage and Trash: They are wastes of residential and commercial areas.
Control
- Sewage Treatment: It removes most of the organic matter and kills the pathogens.
- Effluent Treatment: It removes toxic chemicals.
- Garbage: It can be used to produce manure or generate biogas and manure.
- Trash: It is partly recycled and partly dumped in land fills.
- Electrostatic Precipitators: They remove suspended particles.
- CNG: Use of CNG in vehicles reduces air pollution by automobiles.
Question 32.
(a) Define air pollution. What are the visible indications of air pollution ?
(b) Define acid rain,
(c) Mention any two adverse effects of acid rain. (CCE 2013)
Answer:
(a) Air Pollution: It is addition of particulate matter, gases and vapours into the air that have an adverse effect on humans, animals, vegetation and human assets.
Visible Indications of Air Pollution,
- Occurrence of suspended particulate matter (SPM).
- Common occurrence of asthma, bronchitis and allergic cold in the residents,
- Irritation in eyes, throat and lungs.
- Occurrence of smog during winter causing suffocation and uneasiness.
(b) Acid Rain: Sources of Fresh Water,
- GroundWater. It is brought to the surface through water pumps, dug wells, tube- wells, etc.
- Water Reservoirs. They store run off and rain water.
- Rain. It brings fresh water every where that can be stored.
- Rivers. They develop through melting of snow and formation of springs in the catchment areas. Rivers supply fresh water all through their passage to sea.
(c) Adverse Effects of Acid Rain:
- Corrosion and pitting of marble and limestone structures,
- Killing of vegetation and aquatic life.
Question 33.
(a) Give a schematic representation of oxygen cycle in nature,
(b) State the importance of oxygen cycle in nature.
Or
Explain in how many ways O2 is used up from the atmosphere and how it returns back to the atmosphere.
(CCE 2013, 2014)
Answer:
(a)
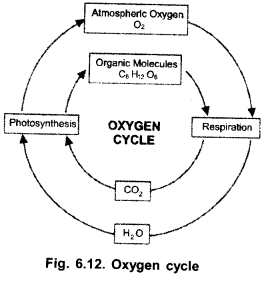
(b) Importance,
- It keeps the oxygen concentration of the atmosphere nearly constant,
- It makes regular supply of oxygen to all organisms for respiration, human beings for combustion, microorganisms for decay and atmosphere for fixation of some nitrogen,
- Oxygen is being regularly added to the atmosphere and aquatic habitats by photosynthetic activity of producers.
Question 34.
(a) In which layer of earth is water available ?
(b) Name the abiotic components of the biosphere.
(c) Name the gas which forms 95-97% of the atmosphere on Mars.
(d) At night which one is warmer, air over sea or air over land ?
( Answer:
(a) Water. It occurs in hydrosphere part of earth which extends from on, in and above earth.
(b) Abiotic Components of Biosphere. Air, water and soil.
(c) CO2
(d) Over sea as water cools down slowly as compared to land.
(e) Coal, petroleum.
Question 35.
(a) How is soil formed ?
(b) Name the four main processes that are part of the water cycle in nature,
(c) Name two compounds formed by nitrogen and oxygen in the nitrogen cycle. (CCE 2013)
Answer:
(a) Soil is formed through two processes of weathering and humification.
Weathering
It is pulverisation of rocks or breaking of rocks into fine particles. There are three types of weathering — physical (atmospheric changes and mechanical forces), chemical and biological. Sun, water, wind and living organisms perform them.
- Sun: It causes expansion of rocks by heating. Cooling causes their contraction. Different parts of rocks expand and contract at different rates. Uneven expansion and contraction produces cracks leading to fragmentation of rocks.
- Water:
- Wetting and Drying: Certain rock components can pick up and lose moisture. They undergo swelling and contraction resulting in fragmentation of rocks.
- Frost Action: Water seeping in cracks would swell up and exert a great pressure if it freezes due to low temperature. The rock would undergo fragmentation.
- Abrasion: Running water carrying rock fragments would break and grind rocks occurring in the pathway. Rain and hail also cause rock breaking.
- Wind: Dust and fine sand carried by wind cause abrasion of the rock surface when wind strikes the same.
- Living Organisms: Lichens secrete chemicals to dissolve minerals from the rock surface. This produces crevices where dust collects. Mosses grow there. They cause deepening of crevices and development of small cracks. Roots of short lived plants widen these cracks. Roots of larger plants cause fragmentation of rocks by entering the cracks and growing in size.
Humification
Partially decomposed organic matter or humus mixes with weathered rock particles to form soil. Humus helps in formation of soil crumbs which are essential for maintaining proper hydration and aeration of soil.
(b) Water Cycle Processes.
- Vaporisation
- Cloud formation
- Rain
- Infiltration and run-off.
(c) Nitrogen oxides (e.g., nitrate), proteins.
Question 36.
(a) Write names of two biologically important compounds that contain both oxygen and nitogen.
(b) (i) Give a schematic representation of nitrogen cycle.
(ii) Name the main processes involved in it. (CCE 2013)
Answer:
(a) Nucleic acids, Proteins.
(b) (i)
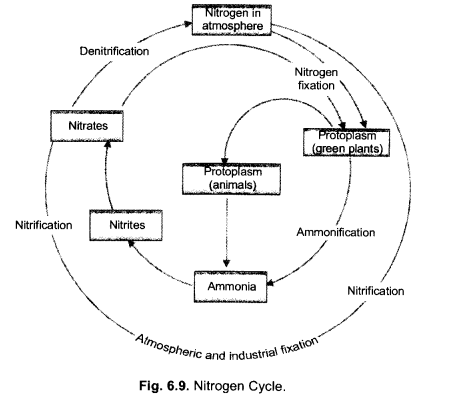
(ii) Main Processes: Processes Involved in Cycling of N2 :
- Nitrogen fixation,
- Nitrogen assimilation
- Decomposition
- Denitrification.
Question 37.
(a) List any two human activities which would lead to an increase in CO2 content of air.
(b) Draw a schematic diagram of carbon cycle in nature. (CCE 2013)
(a)
- Increased combustion of fossil fuels
- Deforestation,
(b)
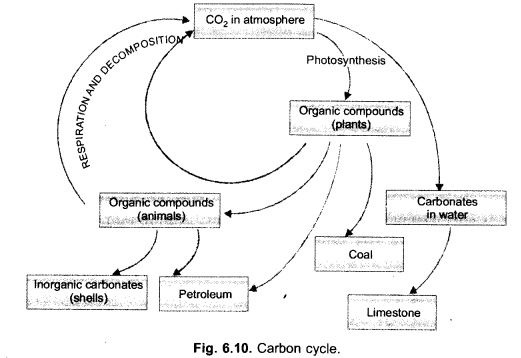
Question 38.
(a) Expand CFC.
(b) Mention one important function of ozone,
(c) What is green house effect ?
(d) Name one green house gas. (CCE 2013)
Answer:
(a) CFC-Chlorofluorocarbon.
(b) Ozone. Filtering harmful high energy ultraviolet
radiations in the atmosphere,
(c) Green House Effect: Green house effect is keeping an area warm by allowing the solar radiations to pass in but preventing long waves to escape due to presence of radiatively active gases and glass panes.
(d) Green House Gas. Carbon dioxide.
Question 39.
(a) What is soil pollution ?
(b) What are the various factors that decide which plants will thrive on a particular kind of soil.
(c) Explain any two factors that cause soil erosion.
(CCE 2013)
Answer:
(a) Soil Pollution: It is adverse alteradon in the soil caused by removal or addition of substances which reduce its fertility and quality.
(b) Soil Factors Controlling Plants Thriving Over It.
Top soil is the upper mineral and humus rich layer of soil that supports vegetation.
Factors:
- Depth of top soil,
- Nutrient and humus content of soil,
- Soil particles,
- Soil pH.
(c) Soil Erosion,
- Absence of vegetation cover,
- Heavy rain, fast run off or strong wind.
Question 40.
(a) What percentage of elemental oxygen is found in earth’s atmosphere and by which process oxygen is returned back to atmosphere.
(b) Represent oxygen cycle that is operating in nature diagrammatically. (CCE 2013)
Answer:
(a)
- 21%
- Photosynthesis.
(b)
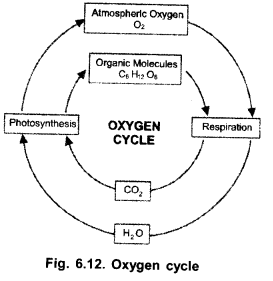
Question 41.
What is biogeochemical cycle ? Draw flow chart of nitrogen cycle in nature. (CCE 2013)
Answer:
Biogeochemical Cycle: The repeated circulation of biogeochemicals or biogenetic nutrients between abiotic and biotic components of the environment is called biogeochemical cycling, e.g., CO2 cycle, N2 cycle, Water cycle, O2 cycle.
Nitrogen Cycle.
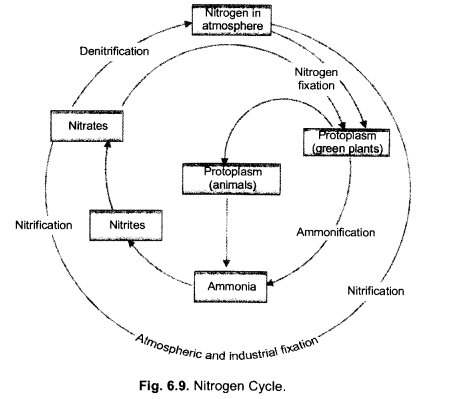
Question 42.
(a) With the help of a neat labelled, diagram, depict the cycling of carbon in nature.
(b) Mention two ways in which carbon dioxide is fixed in nature. (CCE 2013)
Answer:
(a)
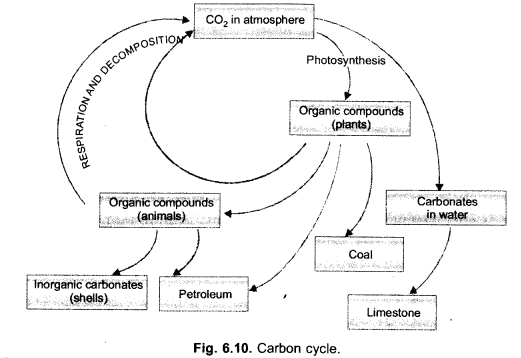
(b)
- Photosynthesis by photoautotrophs
- Animals for building their endoskeleton and shells.
Question 43.
What is meant by air pollution ? Explain any three human activities that cause air pollution. Mention any two harmful effects of air pollution. (C. C.E. 2014)
Answer:
Definition: Air pollution is addition of particulate matter, gases and vapours into the atmosphere that have an adverse effect on humans, animals, vegetation and human assets.
List any three human activities that you think would lead to air pollution.
- Burning of fossil fuels in industries, vehicles and thermal plants.
- Processing industries like textiles, asbestos, flour
- Stone crushing.
Harmful Effects:
Effect on Animal Life: Respiratory disorders, allergies, eye and throat irritation, haemorrhages, frequent diarrhoea, reduced appetite and weakening of bones.
Effect on Plant Life: Chlorosis, necrosis, water soaked areas, defoliation and die back.
Question 44.
(a) Why is the atmosphere essential for life ?
(b) Explain how does atmosphere act as a blanket.
(C.C.E. 2014)
Answer:
(a)
- Oxygen: Atmosphere contains oxygen which is essential for combustion and respiration of most organisms.
- Carbon Dioxide: Atmosphere provides carbon dioxide for photosynthesis of plants.
- Protection: Atmosphere filters out lethal cosmic rays and high energy ultraviolet rays.
- Temperature Buffer: Atmosphere does not allow daytime temperature to rise abnormally nor does it allow night time temperature to fall down drastically. This provides favourable temperature for the living organisms.
- Other Functions: Air currents help in dispersal of spores and other dissemules. Water cycle operates through atmosphere and produces rain to replenish fresh water over land.
(b) Atmosphere or air is a bad conductor of heat. It, therefore, functions as a blanket.
- It does not allow sudden increase in temperature during the daylight hours when sun shines overhead.
- There is no sudden cooling during night. Atmosphere slows down the escape of heat to the outer space from the area of earth under darkness.
- Atmosphere maintains the average temperature of the earth fairly steady not only during the day but throughout the year.
Hope given Previous Year Question Papers for CBSE Class 9 Science Chapter 14 Natural Resources are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.