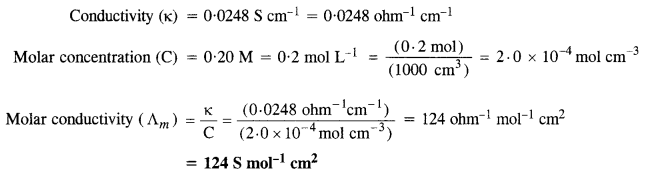
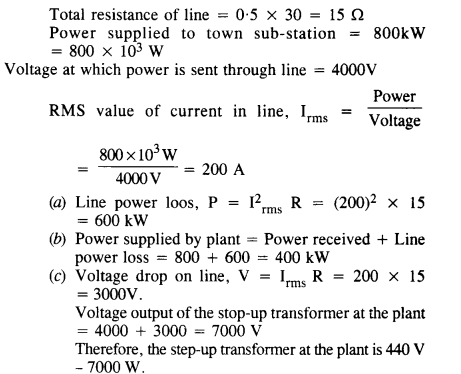
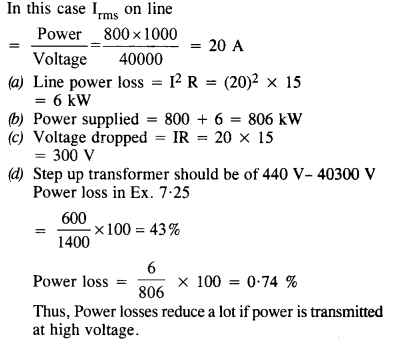
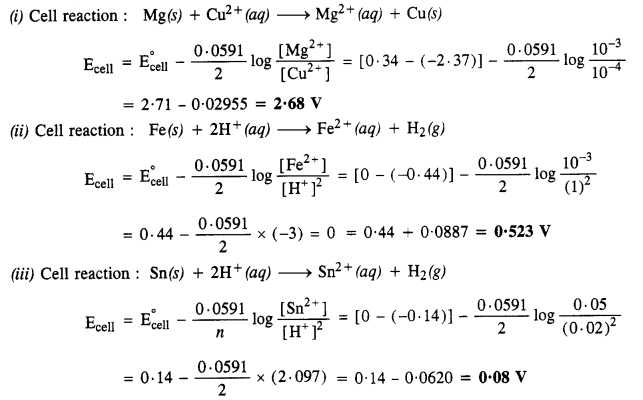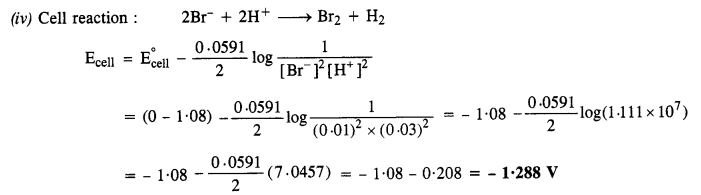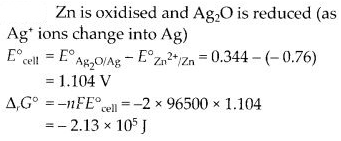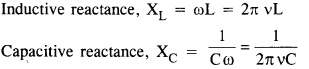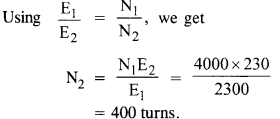NCERT Solutions for Class 6 English Honeysuckle Chapter 9 Desert Animals are part of NCERT Solutions for Class 6 English. Here we have given NCERT Solutions for Class 6 English Honeysuckle Chapter 9 Desert Animals.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 6 |
| Subject | English |
| Chapter | Chapter 9 |
| Chapter Name | Desert Animals |
| Number of Questions Solved | 7 |
| Category | NCERT Solutions |
NCERT Solutions for Class 6 English Honeysuckle Chapter 9 Desert Animals
TEXTUAL QUESTIONS
(Page 117)
Working with the Text
A.
Question 1.
Talk to your partner and say whether the following statements are true or false.
- No animal can survive without water.
- Deserts are endless sand dunes.
- Most snakes are harmless.
- Snakes cannot hear, but they can feel vibrations through the ground.
- Camels store water in their humps.
Solution:
- True
- False
- True
- True
- False
Question 2.
Answer the following questions.
- How do desert animals survive without water ? (1)
- How do mongooses kill snakes ? (6)
- How does the hump of the camels help them to survive when there is no water ? (9)
Solution:
- The desert animals have to find different ways to survive without water. Some animals like gerbils spend the hottest part of the day in cool underground burrows. Some like darkling beetles catch drops of moisture on their legs. Some like camels get the necessary water from the desert plants they eat.
- The reactions of mongooses are so fast that they can dodge each time the snake strikes. They continually make a nuisance of themselves until after a while when the snake gets tired, they quickly dive in for a kill.
- The humps help the camels to survive by acting as storage containers. These humps are full of fat. The fat nourishes the camels in the absence of food and water.
Question :
B. Read the words/phrases in the box. With your partner find their meaning in the dictionary. Fill in the blanks in the following passage with the above words/ phrases.

All animals in forests and deserts struggle to ____________ in ____________ Though most of the animals are ____________ , some are dangerous when . If an ____________ is noticed, they attack or bite to save themselves. They struggle ____________ for food and water. Some animals are called ____________ because they ____________ on other animals.
Solution:
Word Meaning
harsh hard
conditions situations
harmless safe
survive remain alive
intruder unwanted arrival
threatened feel unsafe
predators hunters
prey hunt
continually all the time
All animals in forests and deserts struggle to survive in harsh conditions. Though most of the animals are harmless, some are dangerous when threatened. If an intruder is noticed, they attack or bite to save themselves. They struggle continually for food and water. Some animals are called predators because they prey on other animals.
Speaking
Question :
Look at these sentences.
- Deserts are the driest places on earth.
- Gerbils spend the hottest part of the day in cool underground burrows.
Now form pairs. Ask questions using a suitable form of the word in brackets. Try to answer the questions too.
Do you know
1. Which animal is the ____________ (tall) ?
2. Which animal runs the ____________ (fast) ?
3. Which place on earth is the _ (hot) or the ____________ (cold) ?
4. Which animal is the ____________ (large) ?
5. Which is the ____________ (tall) mountain in the world ?
6. Which is the ____________ (rainy) place on earth ?
7. Which is the ____________ (old) living animal ?
Can you add some questions of your own ?
Solution:
1. tallest
2. fastest
3. hottest … coldest
4. largest
5. tallest
6. most rainy
7. oldest
Students should try to add their own questions to this list.
Thinking about Language
Question :
A. Look at these sentences.
- Most snakes are quite harmless, but a few are poisonous.
- Most snakes lay eggs, but the rattlesnake gives birth to its young.
Now write five sentences like these using ‘most’ and the clues below.
- (90% of) people are honest (10%) are dishonest.
__________________________________________________ - (Lots of) fruit have plenty of sugar, (some) citrus fruit are low in sugar.
__________________________________________________ - (Every soft drink except this one) has lots of empty calories’.
__________________________________________________ - (The majority of) films are romances, (a few) are on other topics.
__________________________________________________ - (A majority of) people agree that he is a good leader, (just a few) disagree.
__________________________________________________
Solution:
1. Most people are honest, but a few are dishonest.
2. Most fruit have plenty of sugar but citrus fruit are low in sugar.
3. Most soft drinks have lots of empty calories but this one is free from them.
4. Most films are romances but a few are on other topics.
5. Most of the people agree that he is a good leader but just a few disagree.
Question :
B. Look at these sentences.
- Animals cannot survive for long without water.
- So desert animals have to find different ways of coping.
The first sentence says what cannot happen or be done ; the second tells us what must, therefore, be done, what it is necessary to do. Complete these sentences using cannot and have to/has to.
1. You ____________ reach the island by land or air ; you go by boat.
2. We ____________ see bacteria with our eyes ; we, look at them through a microscope.
3. He ____________ have a new bicycle now ; he ____________ wait tili next year.
4. Old people often ____________ hear very well ; they ____________ use a hearing aid.
5. Road users ____________ do what they wish ; they ____________ follow the traffic rules.
6. She ____________ accept this decision ; she ____________ question it.
7. you ____________ believe everything you hear ; you ____________ use your own judgement.
Solution:
1. You cannot reach the island by land or air ; you have to go by boat.
2. We cannot see bacteria with our eyes ; we have to look at them through a microscope.
3. He cannot have a new bicycle now ; he has to wait till next year.
4. Old people often cannot hear very well ; they have to use a hearing aid.
5. Road users cannot do what they wish ; they have to follow the traffic rules.
6. She cannot accept this decision ; she has to question it.
7. You cannot believe everything you hear ; you have to use your own judgement.
Writing
Question :
Imagine you are journeying through a desert. Write a couple of paragraphs describing what you see and hear.
Solution:
Once I happened to pass through the deserts of Rajasthan. No vehicle like motor car could take me through the desert and so I had to accept the offer of a camel ride. It was my first chance to ride a camel. In the beginning, it was a little difficult but I got used to it.
We started in the evening because the camel-man told me that during the day the dust storm may become unbearable. He also asked me to fill my bottle with water because for the next six hours, I could not expect to get any water. So equipped with water and other necessary things, I rode the camel determined to cross the small part of the desert and reach the other side to a village. On the way, I saw many duns. Fortunately, the weather remained fine and the night seemed extremely beautiful. Nothing untoward happened on the way and I reached the village before it was dawn.
We hope the NCERT Solutions for Class 6 English Honeysuckle Chapter 9 Desert Animals help you. If you have any query regarding NCERT Solutions for Class 6 English Honeysuckle Chapter 9 Desert Animals, drop a comment below and we will get back to you at the earliest.