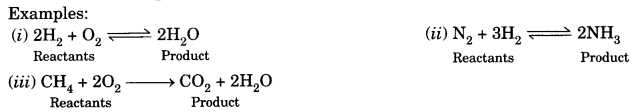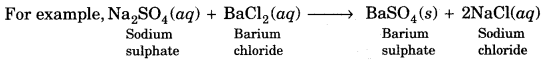
On this page, you will find The Fundamental Unit of Life Class 9 Notes Science Chapter 5 Pdf free download. CBSE NCERT Class 9 Science Notes Chapter 5 The Fundamental Unit of Life will seemingly help them to revise the important concepts in less time.
CBSE Class 9 Science Chapter 5 The Fundamental Unit of Life
The Fundamental Unit of Life Class 9 Notes Understanding the Lesson
1. Robert Hooke: In 1665, he discovered cell in a thin slice of cork (bark of cork tree) by using a self designed microscope. The structure consisted of many little compartments which resembled the structure of a honeycomb. He called boxes as ‘cell’ which is Latin word for ‘a little room’.
2. Leeuwenhoek: In 1674, discovered the free-living cells in pond water for the first time by using an improved microscope.
3. Robert Brown: In 1831, discovered the nucleus in the cell.
4. Piirkinje: In 1839, coined the term ‘protoplasm’ for the fluid substance of the cell.
5. Schleiden (1838) and Schwann (1839): Put forth the cell theory, which said that:
- all the plants and animals are composed of cells and
- cell is the basic unit of life.
6. Virchow: In 1855, expanded the cell theory by suggesting ‘Omni cellula-e-cellula’ which means all cells arise from pre-existing cells.
7. Unicellular organisms: Organisms which have only a single cell, e.gAmoeba, Paramecium, Chlamydomonas, bacteria, etc.
8. Multicellular organisms: Organisms which consist of more than one cell e.g., Plants, animals, fungi, etc.
9. Cell division: It is the process by which a cell divides to form new cells. This supports the fact that, all cells arise from the existing cells.
10. The shape and size of cells are related to the specific function they perform: Amoeba can change its shape as per the conditions or its need whereas for most of the other cases, the cell shape is more or less fixed, e.g., nerve cells have a typical shape.
11. Division of Labour: In all multicellular organisms there is a division of labour. This means that different parts of the body perform different functions. For example, the stomach helps in digestion; blood is pumped by the heart, etc. Division of labour can also be seen within a single cell.
12. Three main parts of a cell: Most cells (excluding bacteria) have three main parts:
- Plasma membrane/Cell Membrane
- Nucleus
- Cytoplasm
13. Plasma Membrane or Cell Membrane
- It is the outermost covering of the cell.
- It separates the contents of the cell from its external environment.
- It is mainly composed of lipids and proteins.
- It is called selectively permeable as it permits the entry and exit of only some materials in and out of the cell.
14. Diffusion: The movement of a substance from a region of its high concentration to the region of its low concentration is called diffusion. Diffusion helps in gaseous exchange between the cells as well as the cell and its external environment.
15. Osmosis: The spontaneous movement of water molecules from a region of its high concentration to the region of its low concentration through a selectively permeable membrane is called osmosis.
Effect on animal cell or a plant cell put into a solution of sugar or salt in water
| Kind of solution | Nature of surrounding medium | Effect on cell | Result |
| Hypotonic solution | Medium surrounding the cell has a higher water concentration than the cell (outside solution is very dilute). | Water will enter the cell by osmosis. | Cell is likely to swell up. |
| Isotonic solution | Medium surrounding the cell has exactly the same water concentration as the cell. | There is no overall movement of water. | Cell will stay the same size. |
| Hypertonic solution | Medium surrounding the cell has a lower concentration of water than the cell (very concentrated solution). | Cell will lose water by osmosis. | Cell will shrink. |
16. Cell Wall
- It is a rigid outer covering which lies outside the plasma membrane.
- It is made of cellulose which provides structural strength to plants.
- The shrinkage or contraction of the contents of the cell away from the cell wall when a living plant cell loses water through osmosis is known as plasmolysis.
17. Nucleus
- It is a dark coloured, spherical or oval, dot-like structure near the centre of each cell.
- It is the control centre of the cell as it controls all the activities of the cell.
- It has a double-layered covering called nuclear membrane.
- The nuclear membrane has pores which allow the transfer of materials from inside the nucleus to its outside, that is, to the cytoplasm.
- The nucleus plays a central role in cellular reproduction (process by which a single cell divides and forms two new cells).
- Nucleus along with the environment directs the chemical activities of the cell to determine the way the cell will develop and the form it will exhibit at maturity.
- Nuclear region of the cell may be poorly defined due to the absence of a nuclear membrane in some organisms like bacteria. Such an undefined nuclear region containing only nucleic acids is called a nucleoid.
18. Chromosomes
- The nucleus contains chromosomes, which are visible as rod-shaped structures only when the cell is about to divide.
- Chromosomes contain information for inheritance of features from parents to next generation in the form of DNA (Deoxyribo Nucleic Acid) molecules.
- Chromosomes are composed of DNA and protein.
19. Genes
Functional segments of DNA are called genes.
Chromatin Material:
- DNA is present as part of chromatin material in the cells which are not dividing.
- Chromatin material is visible as entangled mass of thread-like structures.
- Chromatin material gets organised into chromosomes, when the cell is about to divide.
20. Types of organisms on the basis of the nature of nucleus and nuclear membrane
| Prokaryotes | Eukaryotes |
| (i) Organisms whose cells lack a well defined nuclear membrane. |
(i) Organisms with cells having a well defined nuclear membrane. |
| (ii) They lack membrane bound cell organelles. | (ii) They have membrane bound cell organelles. |
| (iii) Size is generally small (1-10 pm). | (iii) Size is generally large (5-100 pm). |
| (iv) Have a single chromosome. | (iv) Have more than one chromosome. |
20. Cytoplasm
- It is the fluid content enclosed by the plasma membrane.
- It contains many specialised cell organelles.
21. Cell Organelles
Cell organelles are parts of the cell which are specialised for carrying out one or more vital functions, analogous to the organs of the human body.
22. A. Endoplasmic Reticulum (ER)
(i) Consists of a large network of membrane-bound tubes and sheets which appear as long tubules or round or oblong bags (vesicles).
(ii) ER serves as channels for the transport of materials (especially proteins) between various regions of the cytoplasm or between the cytoplasm and the nucleus.
(iii) It also functions as a cytoplasmic framework providing a surface for some of the biochemical activities of the cell.
(iv) ER are of two types:
Rough endoplasmic reticulum (RER) and
Smooth endoplasmic reticulum (SER).
(v) Rough endoplasmic reticulum
- It looks rough as it has particles called ribosomes attached to its surface. Ribosomes are the site of protein synthesis.
(vi) Smooth endoplasmic reticulum
- It helps in the manufacture of fat molecules, or lipids, important for cell function.
- Some proteins and lipids made by SER help in building the cell membrane and this process is known
as membrane biogenesis. - Helps in detoxifying many poisons and drugs in the liver cells of the group of vertebrates.
B. Golgi Apparatus
- First described by Camillo Golgi.
- It has membrane-bound vesicles arranged approximately parallel to each other in stacks called cisterns.
- They constitute another portion of a complex cellular membrane system as their membranes often have connections with the membranes of ER.
- Its functions include storage, modification and packaging of products in vesicles.
- Golgi apparatus packages and dispatches the material synthesised near the ER to various targets inside and outside the cell.
- They are also involved in the formation oflysosomes.
C. Lysosomes
- They are membrane-bound sacs filled with digestive enzymes made by RER.
- They are waste disposal system of the cell as they help to keep the cell clean by digesting any foreign material as well as worn-out cell organelles.
- Lysosomes have powerful digestive enzymes capable of breaking down all organic materials.
- Lysosomes are also known as the ‘suicide bags’ of a cell because if the cell gets damaged during disturbance in cellular metabolism, the lysosomes may burst and its enzymes digest their own cell.
D. Mitochondria
- Also known as the powerhouses of the cell as the energy required for various chemical activities needed for life is released by mitochondria in the form of Adenosine triphosphate – ATP (ATP is known as the energy currency of the cell).
- Mitochondrion is a double-membrane structure whose outer membrane is very porous while the inner
membrane is deeply folded to form cristae. Cristae are folds which create a large surface area for ATP’ generating chemical reactions. - Mitochondria have their own DNA and ribosomes so they can make some of their own proteins.
E. Plastids
- Plastids are present only in plant cells and are of two types – chromoplasts (coloured plastids) and leucoplasts (white or colourless plastids).
- Chlorophyll containing plastids are known as chloroplasts and help in photosynthesis.
- Leucoplasts store starch (amyloplast), oils (elaioplasts) and protein granules (aleuroplasts).
- The plastids internally consist of numerous membrane layers embedded in a material called the stroma.
- Plastids have their own DNA and ribosomes so they can make some of their own proteins.
F. Vacuoles
- Vacuoles are storage sacs for solid or liquid contents like amino acids, sugars, various organic acids and some proteins.
- Small-sized vacuoles are present in animal cells while plant cells have very large vacuoles. A large central vacuole may occupy 50-90% of the cell volume in some plant cells.
- The vacuoles are full of cell sap and provide turgidity and rigidity to the cell in plant cells.
- Food vacuole found in Amoeba contains the food items that the Amoeba has consumed.
- Contractile vacuole found in some unicellular organisms help in expelling excess water and some wastes f from the cell.
Class 9 Science Chapter 5 Notes Important Terms
Unicellular organisms: They are single-celled organisms, e.g., Amoeba, Paramecium, Chlamydomonas, bacteria, etc.
Multicellular organisms: They are composed of more than one cell, e.g., plants, animals, fungi, etc. Diffusion: The movement of a substance from a region of its high concentration to the region of its low concentration is called diffusion.
Osmosis: The spontaneous movement of water molecules from a region of its high concentration to the region of its low concentration through a selectively permeable membrane is called osmosis.
Hypotonic solution: If the medium surrounding the cell has a higher water concentration than the cell i.e., outside solution is very dilute, then it is called a hypotonic solution.
Isotonic solution: If the medium surrounding the cell has exactly the same water concentration as the cell, then it is called isotonic solution.
Hypertonic solution: If the medium surrounding the cell has a lower concentration of water than the cell i.e., very concentrated solution, then it is called hypertonic solution.
Plasmolysis: The shrinkage or contraction of the contents of the cell away from the cell wall when a living plant cell loses water through osmosis is known as plasmolysis.
Genes: Functional segments of deoxyribonucleic acid (DNA) are called genes.
Prokaryotes: The single celled organisms which lack a well-defined nuclear membrane are called prokaryotes.
Eukaryotes: The single celled or multicellular organisms which have a well defined nuclear membrane are called eukaryotes.
Membrane biogenesis: Some proteins and lipids made by SER help in building the cell membrane and this process is known as membrane biogenesis.
Amyloplast: The starch containing leucoplasts are called amyloplast.
Elaioplast: The oil containing leucoplasts are called elaioplasts.
Aleuroplast: The protein containing leucoplasts are called aleuroplasts.
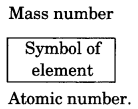
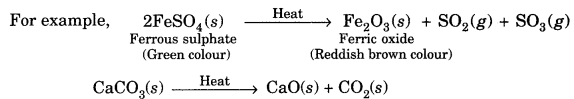
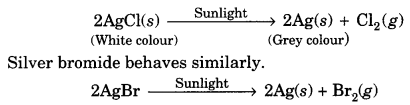
 On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT
On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT