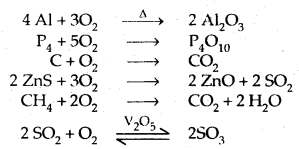
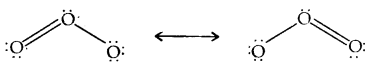
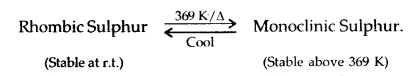
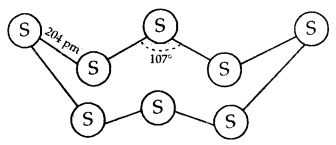
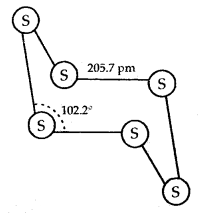
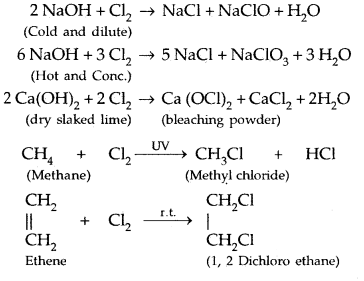
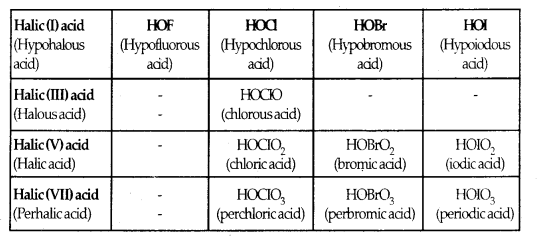
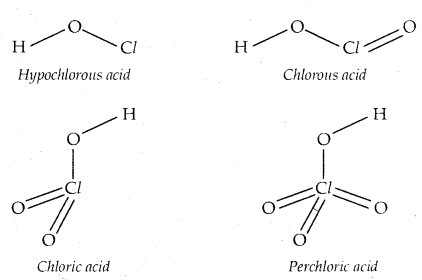
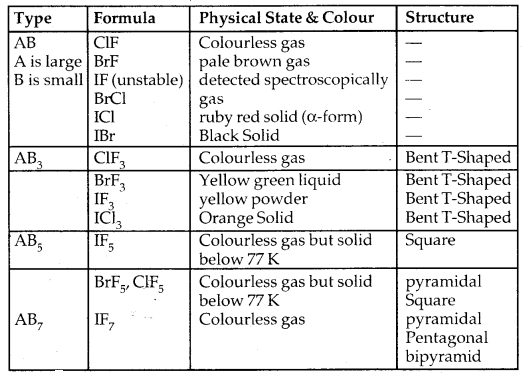
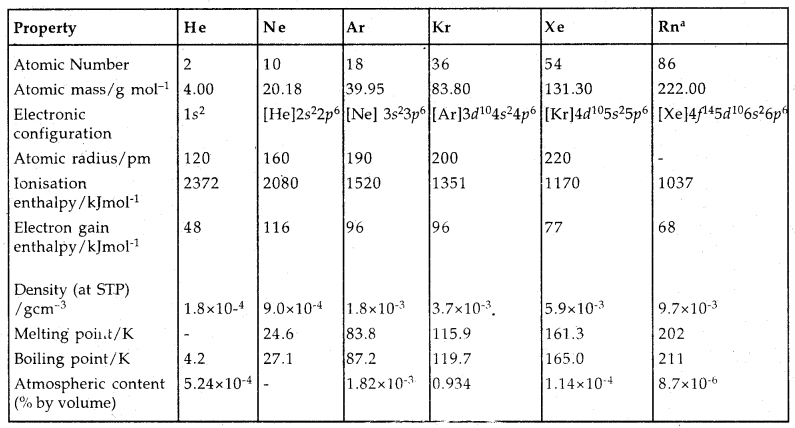
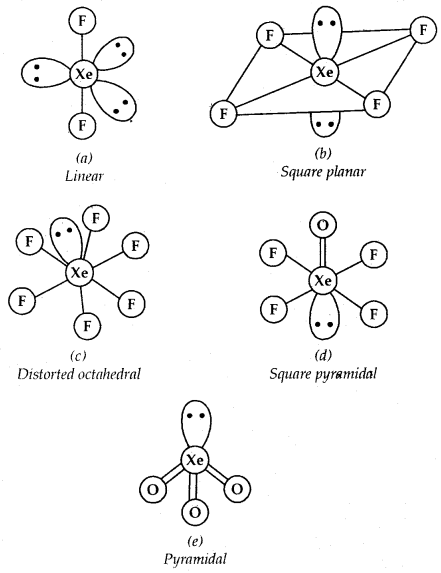
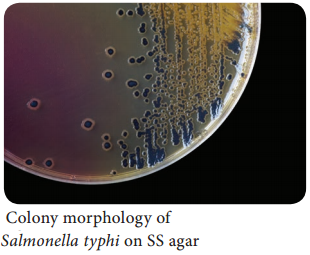
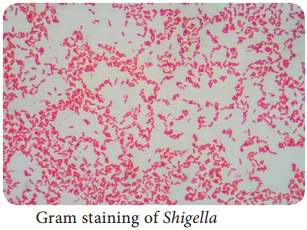
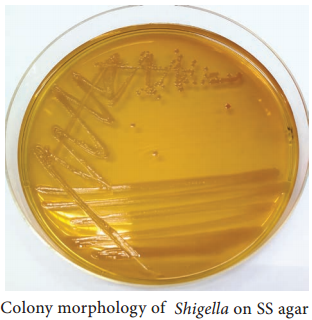
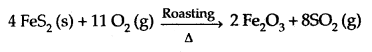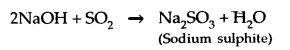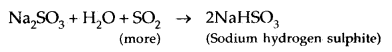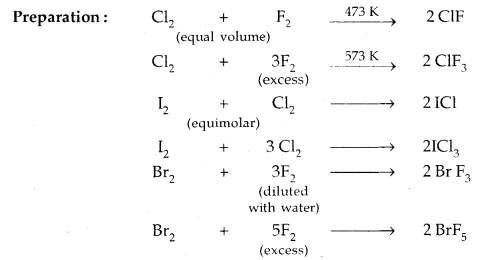Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Treponema Pallidum of Medical Bacteriology
Treponema pallidum is included in the family Spirochaetaceae. They are slender spirochaetes with fine spirals having pointed ends. Some of them are pathogenic for humans, while others are normal flora in mouth and genitalia.
These pathogens are strict parasites and the diseases caused by Treponema are called Treponematoses. Treponema pallidum is the causative agent of syphilis. The name Treponema pallidum is derived from Greek words, which means, Trepos – Turn Nema – Thread and palladium – Pale staining.
Morphology
It is thin, delicate spirochete with tapering ends, about 10µm long and 0.1-0.2 µm wide. It has about ten regular spirals, which are sharp and angular, at regular intervals of about 1µm. They are actively motile (endoflagella), exhibiting rotation around the long axis, backward and forward movements and flexion of the
whole body.
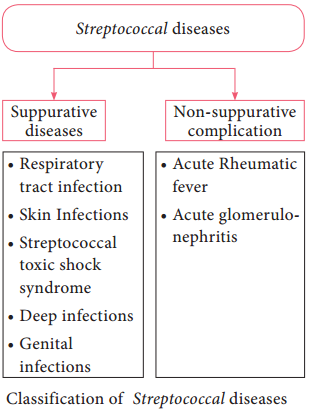
It cannot be seen under light microscope and does not take ordinary bacterial stains. It can be seen under the dark ground (Figure 7.22) or phase contrast microscope.It can be stained by silver impregnation method.
Culture
- Pathogenic Treponema cannot be grown in artificial culture media.
Pathogenesis
Source of infection – Human beings (patients)
Mode of transmission – Sexual contact
Site of entry – Through minute abrasions/cuts on skin or mucosa
Incubation period – 10 – 90 days
- Treponema pallidum causes venereal syphilis, which is acquired by sexual contact. The pathogen enters the human body through cut on the skin or mucosa of genital areas.
- The clinical disease sets in after an incubation period of about a month. There are 3 clinical stage of venereal syphilis, namely – primary, secondary and tertiary syphilis.
Primary syphilis
- A papule appears on the genital area that ulcerates, forming a chancre of primary syphilis called hard chancre.
- The chancre is covered by thick exudates, very rich in spirochetes.
- The regional lymph nodes are swollen, discrete, rubbery and non – tender.
- Even before the chancre appears, the spirochetes spread from the site of entry into the lymph and bloodstream, so the patient may be infectious during the late incubation period.
- The chancre invariably heals within 10-40 days, even without treatment, leaving a thin scar.
Secondary syphilis
- Secondary syphilis sets in 1-3 months after the primary lesion heals. During this interval, the patient is asymptomatic.
- The secondary lesions are due to widespread multiplication of the spirochetes and dissemination through the blood.
- Secondary syphilis is characterized by appearance of papular skin rashes, mucous patches in the oropharynx and condylomata (a raised growth on the skin resembling a wart).
- The lesions are abundant in spirochetes and the patient is most infectious during the secondary stage.
- There may be retinitis (inflammation of the retina of the eye), meningitis, periostitis, and arthritis.
- Secondary lesions usually undergo spontaneous healing, in some cases taking as long as 4 or 5 years.
- After the secondary lesions disappear, there is a period of dormant known as latent syphilis the patient does not show any clinical symptoms but with positive serology.
Tertiary syphilis
- After several years, manifestations of tertiary syphilis appear. These consist of cardiovascular lesions including aneurysms (enlargement of an artery), gummata (a small rubbery granuloma that has a necrotic centre) and meningovascular manifestations. Tertiary lesions contain few spirochetes.
- In few cases, neurosyphilis such as tabesdorsalis or general paralysis of the insane develops. These are known as late tertiary or quaternary syphilis.
Congenital syphilis
In congenital syphilis, the infection is transmitted from mother to fetus by crossing the placental barrier.
Non – Venereal syphilis
It may occur in doctors or nurses due to contact with patients lesion during examination. The primary chancre occurs usually on the fingers.
Laboratory Diagnosis
The diagnosis of syphilis includes
- Demonstration of Treponemes
- Serological tests
Specimen:
Exudates are collected from the chancre. Blood (serum) is collected for serology.
Demonstration of Treponemes
a. Dark ground microscopy:
The wet film is prepared with exudates and examined under dark ground microscope. Under dark field examination Treponema pallidum appears motile spiral organism.
b. Treponemes in tissues:
It can be demonstrated by silver impregnation method of staining.
Serological tests
Non – Treponemal tests – In the standard tests for syphilis includes;
- VDRL – Venereal Diseases Research Laboratory test.
- RPR – Rapid Plasma Reagain (Figure 7.23).
VDRL or RPR tests are used for serological screening for syphilis and more useful for the assessment of cure following treatment.
Treponemal Tests:
The treponemal tests includes
- TPHA – Treponema pallidum hemagglutination assay
- FTA – ABS – Fluorescent treponemal antibody absorption test.
These two tests are used to confirm the diagnosis.
Treatment and Preventive Measure
In early syphilis
- Benzathine benzyl penicillin, 24 lakhs units intramuscularly in a single dose.
- Alternatively, doxycycline 100 mg twice a day orally for 15 days.
In late syphilis
Benzathine benzyl penicillin 24 lakhs units, intramuscularly once weekly for 3 weeks.
- Avoiding sexual contact with an infected individual.
- Use of sex barriers (condoms).