Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Types of Respiration
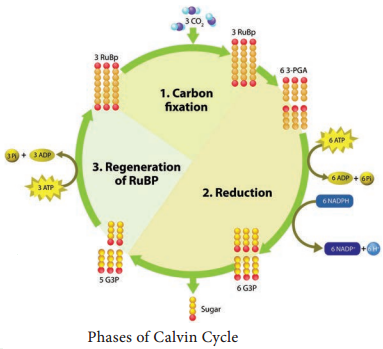
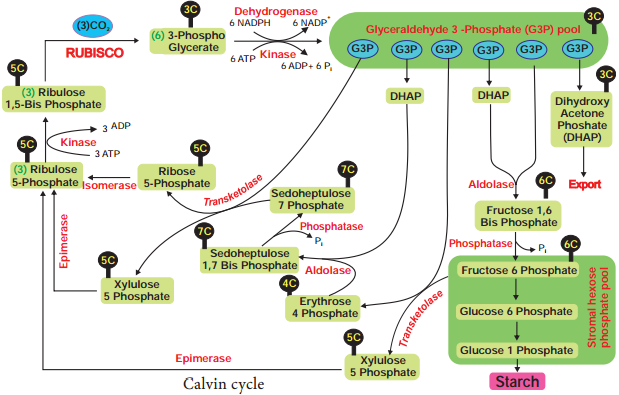
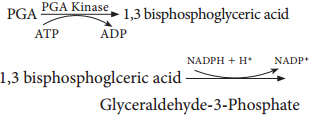
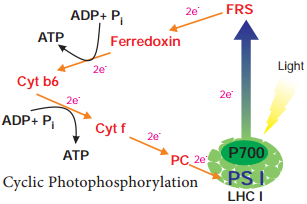
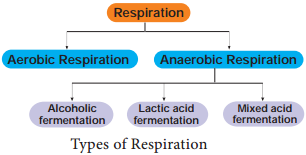
Respiration is classified into two types as aerobic and anaerobic respiration (Figure 14.4)
Aerobic Respiration
Respiration occurring in the presence of oxygen is called aerobic respiration. During aerobic respiration, food materials like carbohydrates, fats and proteins are completely oxidised into CO2, H2O and energy is released. Aerobic respiration is a very complex process and is completed in four major steps:
- Glycolysis
- Pyruvate oxidation (Link reaction)
- Krebs cycle (TCA cycle)
- Electron Transport Chain (Terminal oxidation).
Anaerobic Respiration
In the absence of molecular oxygen glucose is incompletely degraded into either ethyl alcohol or lactic acid (Table 14.1). It includes two steps:
- Glycolysis
- Fermentation
|
Aerobic Respiration |
Anaeorbic Respiration |
| 1. It occurs in all living cells of higher organisms | 1. It occurs yeast and some bacteria |
| 2. It requires oxygen for breaking the respiratory substrate | 2. Oxygen is not required for breaking the respiratory substrate |
| 3. The end products are CO2 and H2O | 3. The end products are alcohol, and CO2 (Or) lactic acid |
| 4. Oxidation of one molecule of glucose produces 36 ATP molecules | 4. Only 2 ATP molecules are produced |
| 5. It consists of four stages-glycolysis, link reaction, TCA cycle and electron transport chain | 5. It consists of two stages-glycolysis and fermentation |
| 6. It occurs in cytoplasm and mitochondria | 6. It occurs only in cytoplasm |
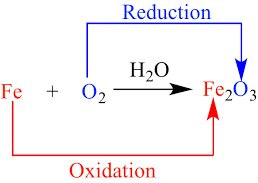
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.
Because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. As such, electron-transfer reactions are also called oxidation-reduction reactions, or simply redox reactions.
Remember that although redox reactions are common and plentiful, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to another. Both reactions above are examples of oxidation-reduction reactions.
Oxidation-reduction reaction, also called redox reaction, any chemical reaction in which the oxidation number of a participating chemical species changes.
Simple Redox Reactions
- Write the oxidation and reduction half-reactions for the species that is reduced or oxidized.
- Multiply the half-reactions by the appropriate number so that they have equal numbers of electrons.
- Add the two equations to cancel out the electrons. The equation should be balanced.
What is the only pattern for redox reactions? Describe how redox reactions always occur. Redox reactions always occur together; if one reactant in a chemical reaction is oxidized, then another reactant must be reduced.
Double-replacement reactions such as the one below are not redox reactions because ions are simply recombined without any transfer of electrons. Note that the oxidation numbers for each element remain unchanged in the reaction.
The batteries which are used for generating DC current use redox reaction to produce electrical energy. Batteries also called as electrochemical cells used in our day-to-day life are also based on redox reactions. For example, storage cells which are used in vehicles to supply all the electrical needs of the vehicles. These reactions cannot be reversed once they occur, because the product escapes the surface immediately.
Oxidation reactions may be exothermic or endothermic. Eg. All combustion reactions are exothermic and ionisation reaction giving positive ions are endothermic.
The key difference between redox and nonredox reactions is that in redox reactions, the oxidation state of some chemical elements changes from one state to another state whereas, in nonredox reactions, the oxidation states of chemical elements do not change.