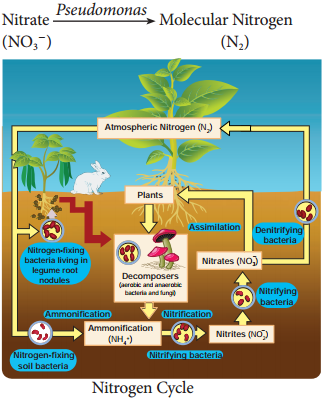
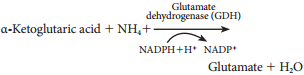
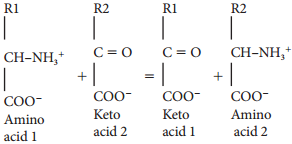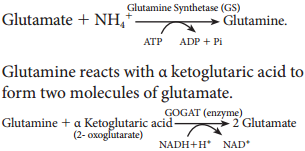Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Photo Chemical Phase of Light Reaction
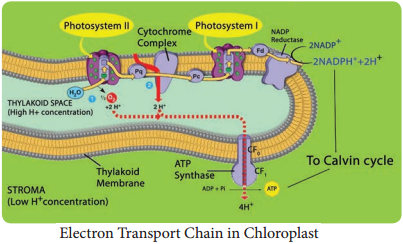
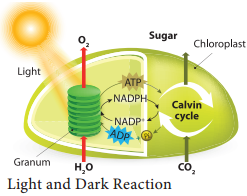
In this phase electrons pass through electron carrier molecules and generate assimilatory powers ATP and NADPH + H+. Splitting of water molecule generates electrons replacing electrons produced by the light.
Photolysis of Water
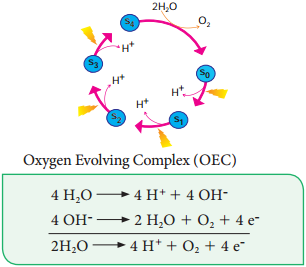
The process of Photolysis is associated with Oxygen Evolving Complex (OEC) or water splitting complex in pigment system II and is catalysed by the presence of Mn++ and Cl–. When the pigment system II is active it receives light and the water molecule splits into OH– ions and H+ ions. The OH– ions unite to form water molecules again and release O2 and electrons (Figure 13.11).
Electron Transport Chain of Chloroplast
Electron Transport Chain in each photosystem involves four complexes:
Core Complex (CC):
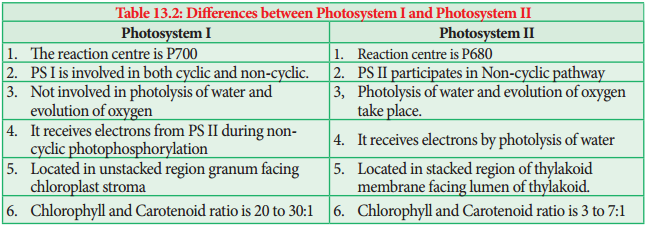
CC I in PS I the reaction centre is P700, CC II in PS II the reaction centre is P680
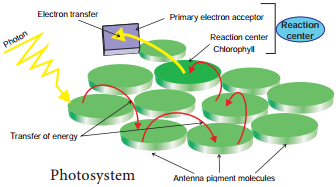
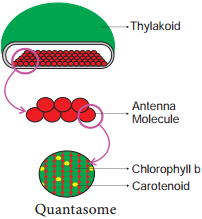
Light Harvesting Complex or Antenna
Complex (LHC):
Two types: LHC I in PS I and LHC II in PS II.
Cytochrome b6 f Complex:
It is the non-pigmented protein complex connecting PS I and PS II. Plastoquinone (PQ) and Plastocyanin (PC) are intermediate complexes acting as mobile or shuttle electron carriers of Electron Transport Chain. PQ acts as shuttle between PS II and Cytochrome b6 – f complex and PC connects.
Cytochrome b6-f and PS I Complex
ATPase complex or Coupling Factor:
It is found in the surface of thylakoid membrane. This complex is made up of CF1 and CF0 factors. This complex utilizes energy from ETC and converts ADP and inorganic phosphate (Pi) into ATP (Figure 13.12).