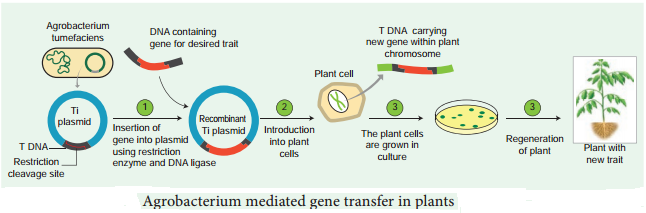
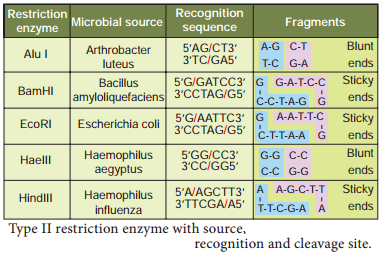
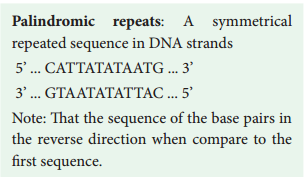
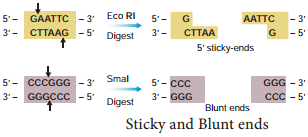
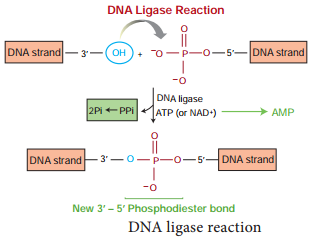
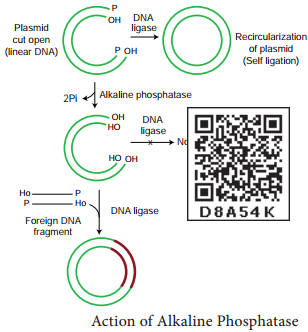
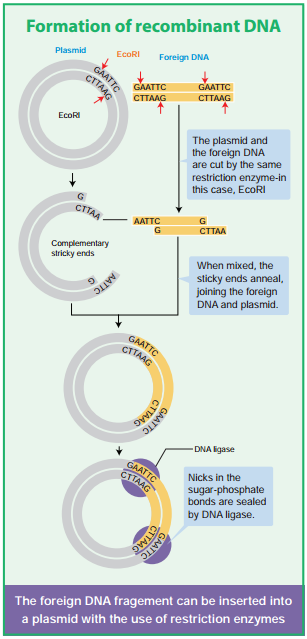
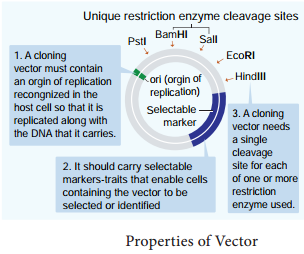
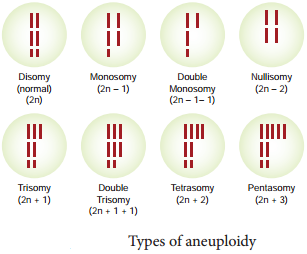
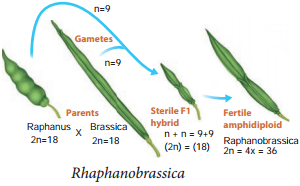
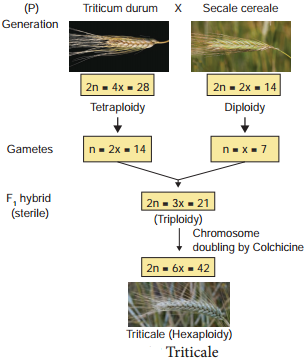
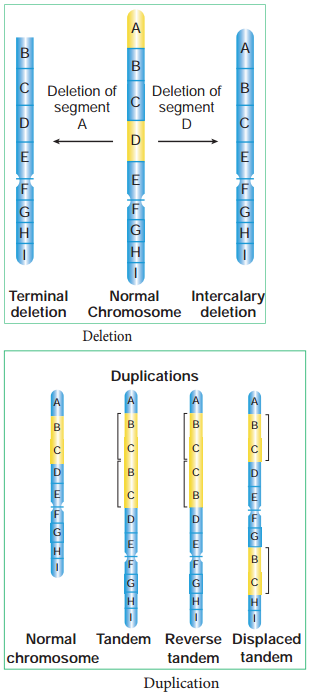
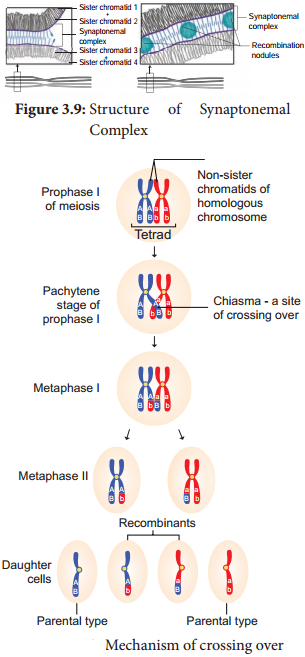
Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Basic Concepts In Plant Disuse Culture
Growing plant protoplasts, cells, tissues or organs away from their natural or normal environment, under artificial condition, is known as Tissue Culture. It is also known as in vitro (In vitro is a Latin word, it means that – in glass or in test-tube) growth of plant protoplasts, cells, tissues and organs. A single explant can be multiplied into several thousand plants in a short duration and space under controlled conditions.
Tissue culture techniques are often used for commercial production of plants as well as for plant research. Plant tissue culture serves as an indispensable tool for regeneration of transgenic plants. Apart from this some of the main applications of Plant tissue culture are clonal propagation of elite varieties, conservation of endangered plants, production of virus-free plants, germplasm preservation, industrial production of secondary metabolites. etc., In this chapter let us discuss the history, techniques, types, applications of plant tissue culture and get awareness on ethical issues.
Gottlieb Haberlandt (1902) the German Botanist proposed the concept Totipotency and he was also the first person to culture plant cells in artifiial conditions using the mesophyll cells of Lamium purpureum in culture medium and obtained cell proliferation. He is regarded as the father of tissue culture.
Basic concepts of Tissue Culture
Basic concepts of plant tissue culture are totipotency, diffrentiation, dediffrentiation and rediffrentiation.
Totipotency
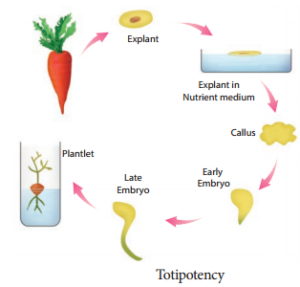
The property of live plant cells that they have the genetic potential when cultured in nutrient medium to give rise to a complete individual plant.
Differentiation
The process of biochemical and structural changes by which cells become specialized in form and function.
Rediffrentiation
The further diffrentiation of already differentiated cell into another type of cell. For example, when the component cells of callus have the ability to form a whole plant in a nutrient medium, the phenomenon is called redifferentiation.
Dediffrentiation
The phenomenon of the reversion of mature cells to the meristematic state leading to the formation of callus is called dediffrentiation. These two phenomena of rediffrentiation and dedifferentiation are the inherent capacities of living plant cells or tissue. This is described as totipotency.