 On this page, you will find Periodic Classification of Elements Class 10 Notes Science Chapter 5 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 5 Periodic Classification of Elements will seemingly help them to revise the important concepts in less time.
On this page, you will find Periodic Classification of Elements Class 10 Notes Science Chapter 5 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 5 Periodic Classification of Elements will seemingly help them to revise the important concepts in less time.
CBSE Class 10 Science Chapter 5 Periodic Classification of Elements
Periodic Classification of Elements Class 10 Notes Understanding the Lesson
1. At present, 118 elements are known to us. All these have different properties. Out of these 118, only 98 are naturally occurring. These elements have different characteristic properties, so it is very difficult to study these elements individually. Scientists made several attempts to classify elements according to their properties.
2. Dobereiner’s Triads (1817): He identified some triads (groups having three elements). Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was the average of the atomic masses of the other three elements. For example,
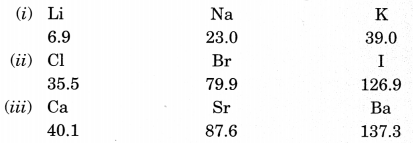
Dobereiner could identify only three triads from the elements known at that time. Hence, this system of classification into triads was not found to be useful.
3. Newland’s Law of Octaves (1866): He arranged the known elements in the order of increasing atomic masses. He found that every eighth element had properties similar to that of the first like the musical note. It is known as Newlands law of octaves.
| sa (do) | re (re) | ga (mi) | ma (fa) | pa (so) | da (la) | ni (ti) |
| H | Li | Be | B | C | N | O |
| F | Na | Mg | A1 | Si | P | s |
| Cl | K | Ca | Cr | Ti | Mn | Fe |
| Co and Ni | Cu | Zn | Y | In | As | Se |
| Br | Rb | Sr | Ce and La | Zr | – | – |
4. Limitations
- Law of octaves was applicable only upto calcium.
- Discovery of Noble gases disturbed the octaves.
- Octaves worked well only for lighter elements.
- Properties of new elements could not fit in it.
5. Mendeleev’s Periodic Table
Mendeleev’s Periodic Law: Physical and chemical properties of elements are the periodic function of their atomic masses.
Mendeleev’s periodic table is based on the chemical properties of elements. Mendeleev’s periodic table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’.
6. Achievements of Mendeleev’s Periodic Table: 63 elements were known at the time of classification.
- Elements with similar properties could be grouped together.
- Mendeleev left some gaps in his periodic table. Mendeleev boldly predicted the existence of some elements that had not been discovered at that time.
- Noble gases discovered, could be placed without disturbing the existing order.
Remember
Scandium, gallium and germa-nium have properties similar to Eka-boron, Eka-aluminium and Eka-silicon respectively.
7. Limitations of Mendeleev’s Classification
- The position of hydrogen in the table was not certain because it could be placed in the group of alkali metals as well as in halogens.
- Isotopes of elements were placed in the same position in the table though according to their atomic masses, they should have been placed in different positions.
- Certain elements of higher atomic mass preceed those with lower atomic mass. For example, tellurium (atomic mass 127.6) precedes iodine (atomic mass 126.9). Iodine was placed after tellurium though it had lower atomic mass because Iodine had properties similar to bromine and not selenium.
8. The Modern Periodic Table
Henry Moseley (1913) exhibited that the atomic number of an element is a more fundamental property than its atomic mass. Mendeleev’s periodic law was modified and atomic number was adopted as the basis of the modern periodic table.
9. The Modern Periodic Law states that:
The physical and chemical properties of the elements are the periodic function of their atomic numbers.
It means that if the elements are arranged in order of increasing atomic numbers, the elements with similar properties recur after regular intervals. Many new forms of periodic table have been proposed in recent times with modern periodic law as the guiding principle, but the general plan of the table remains the same as proposed by Mendeleev. The most commonly known periodic table is the Long form of the periodic table.
- Modern periodic table contains 18 vertical columns known as groups and 7 horizontal rows known as periods.
- Elements in a group have the same number of valence electrons.
- Number of the shells increases as we go down the group.
- Elements in a period have same number of shells.
- Number of elements placed in a particular period depends upon the fact that how electrons are filled into various shells.
- Maximum number of electrons that can be accommodated in a shell depends on the formula 2n2 where n is the number of the given shell. For example, in K shell the number of electrons is 2 x (1)2 = 2 in the first period, L shell – 2 x (2)2 = 8 elements in the second period.
- Position of the element in the periodic table tells about its reactivity.
10. Trends in the Modern Periodic Table
Valency: Number of valence electrons present in the outermost shells. Valency remains the same down a group but changes across a period.
Atomic Size: Atomic size refers to radius of an atom.
Atomic size or radius decreases in moving from left to right along a period due to increase in nuclear charge. Atomic size increases down the group because new shells are being added as we go down the group.
Metallic Character: Metallic character means the tendency of an atom to lose electrons.
Metallic character decreases across a period because the effective nuclear charge increases, that means the tendency to lose electrons decreases. Metals are electropositive as they tend to lose electrons while forming bonds.
Class 10 Science Chapter 5 Notes Important Terms
Atomic number of an element is equal to the number of protons in the nucleus of its neutral atom.
Atomic number = Number of protons = Number of electrons
Electronic configuration corresponds to the distribution of electrons in the different shells.
Element is a chemical substance that cannot be decomposed by chemical means into simple substances. It contains the same kind of atoms.
Groups are the vertical rows in the periodic table.
Periods are the horizontal rows in the periodic table.
Periodic table is a tabular arrangement of elements in groups (vertical columns) and periods (horizontal rows) highlighting the regular trends in physical and chemical properties.
Shell is a region around the nucleus in an atom where electron revolves.
Valence shell is the outermost shell of an atom.
Periodicity. The properties which reoccur after a regular intervals in a periodic table are called periodic properties and the phenomenon is called periodicity of element.
Modern. Modern periodic law can be stated as follows:
“Physical and chemical properties of elements are a periodic function of their atomic number”.
 On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT
On this page, you will find Metals and Non-metals Class 10 Notes Science Chapter 3 Pdf free download. CBSE NCERT 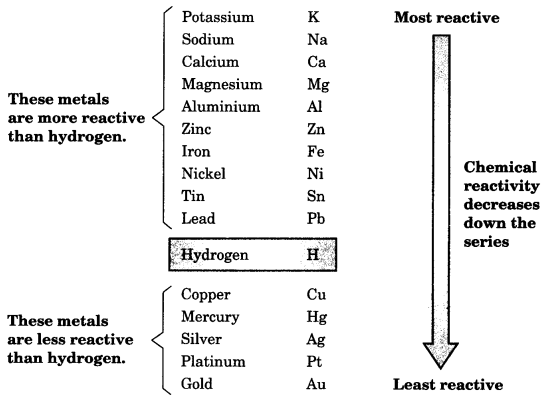
 On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT
On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT 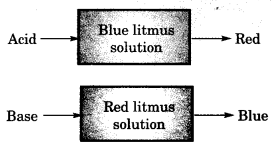
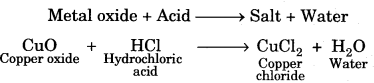
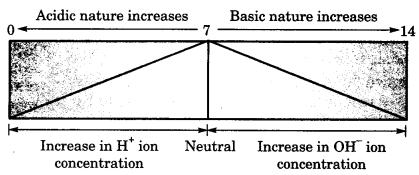
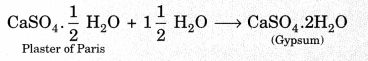
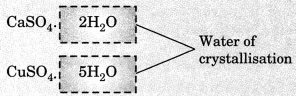
 On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT
On this page, you will find Chemical Reactions and Equations Class 10 Notes Science Chapter 1 Pdf free download. CBSE NCERT