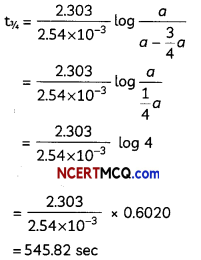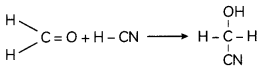Students can access the CBSE Sample Papers for Class 12 Chemistry with Solutions and marking scheme Term 2 Set 5 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 12 Chemistry Term 2 Set 5 with Solutions
Time Allowed: 2 Hours
Maximum Marks: 35
General Instructions:
- There are 12 questions in this question paper with internal choice.
- SECTION A – Q. No. 1 to 3 are very short answer questions carrying 2 marks each.
- SECTION B – Q. No. 4 to 11 are short answer questions carrying 3 marks each.
- SECTION C- Q. No. 12 is case based question carrying 5 marks.
- All questions are compulsory.
- Use of log tables and calculators is not allowed.
Section – A
(Section A-Question No 1 to 3 are very short answer questions carrying 2 marks each.)
Question 1.
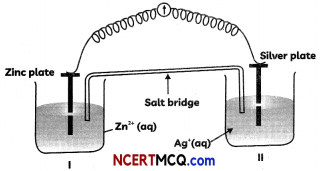
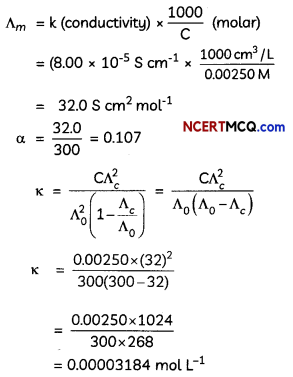
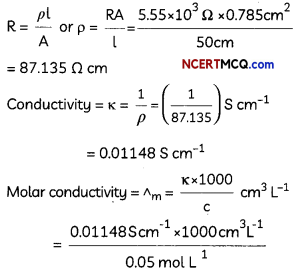
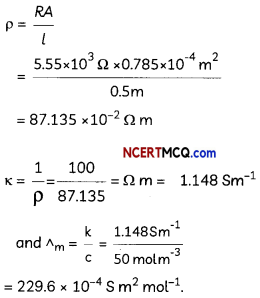
Express the relation among cell constant, resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity? (2)
Answer:
κ = \(\frac{1}{R} \times\left(\frac{l}{A}\right)\)
Where κ = Conductivity
\(\frac{l}{A}\) = Cell Constant
R = Resistance
Am = \(\frac{\kappa \times 1000}{M}\)
Where Am = Molar conductivity
κ = Conductivity
M = Molarity of Solution
Related theory-Conductivity: Conductivity of a solution is defined as the conductance of a solution of 1 cm length and having 1 sq. cm as the area of cross-section. It is represented by K. Its unit is S cm-1
Molar conductivity: Molar conductivity of a solution at a dilution V is the conductance of all the ions produced from one mole of the electrolyte dissolved in V cm3 of the solution when the electrodes are 1 cm apart and the area of the electrodes is so large that the whole of the solution is contained between them. It is represented by ∧m. Its unit is S cm2 mol-1
Conductivity and molar conductivity of electrolytes increase with increasing temperature.
![]()
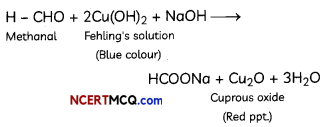
Question 2.
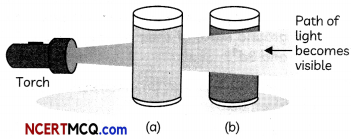
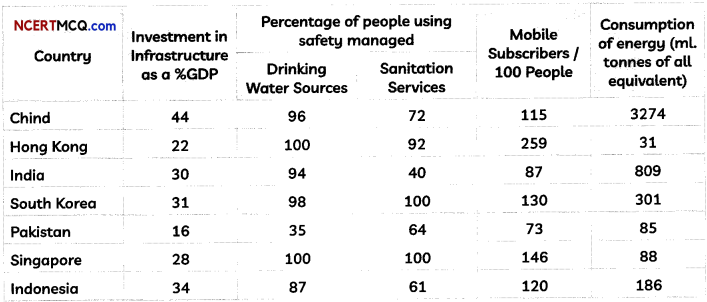
Study the change below after addition of Tollen’s reagent to the test tube and report the kind of compound present in the test tube ? (2)
Answer:
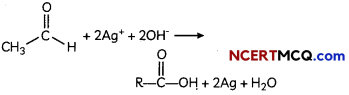
The compound in the test tube is an aldehyde. When an aldehyde is introduced to the Tollen’s reagent,
Aldehyde is oxidized by the Tollen’s reagent and forms a carboxylic acid. The silver ions present in the Tollen’s reagent are reduced into metallic silver. This is because the reduction of the silver ions into metallic silver form a silver mirror on the test tube This reaction can be written as follows:
Question 3.
Write short notes on the following (any two) (2)
(A) Gabriel phthalimide synthesis
Answer:
Gabriel phthalimide synthesis:
It is used for mainly aliphatic primary amine preparation. Phthalimide reacts with ethanolic potassium hydroxide and produces potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis gives corresponding primary amine.
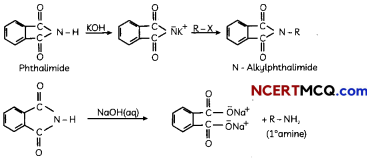
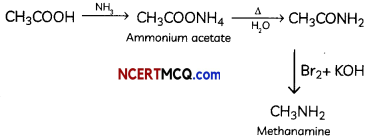
(B) Hoffmann’s bromamide degradation
Answer:
Hoffmann’s Bromamide synthesis:
When amide is treated with Br2 in aqueous or ethanolic solution of NaOH, degradation of amide results in formation of primary amine.
Primary amine formed contains one carbon less than the number of carbon atoms in amide.

![]()
(C) Carbyl amine reaction
Answer:
When aliphatic/aromatic primary amines are heated with chloroform and ale KOH, foul-smelling alkyl isocyanides or carbyl amines are obtained. Secondary or tertiary amines do not give this test.
![]()
Related Theory:
Students should write the chemical reaction involved in every name reaction.
SECTION – B
(Section B-Question No 4 to 11 are short answer questions carrying 3 marks each.)
Question 4.
(A) Give simple tests to distinguish between the following pairs of compounds:
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
Answer:
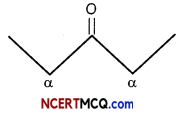
(A) (i) Pentan-2-one and Pentan -3 -one:
Iodoform Test – Pentan – 2- one contains CH3CO– group and as such it will give iodoform test with NaOl [NaOH + I2] while no such group is there in pentan -3- one and so it will not give iodoform test.
![]()
(ii) Benzaldehyde is an aromatic aldehyde while acetophenone is a methyl ketone. These may be distinguished as follows.
Iodoform test: Acetophenone, due to the presence of CH3CO– group, will give iodoform test with NaOl (NaOH + I2), while benzaldehyde will not give this test.
![]()
C6H5CHO + NaOl → No yellow ppt
![]()
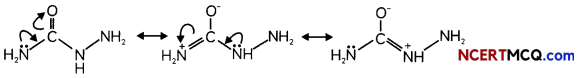
(B) There are two – NH2 groups in semicarbazide. However, only one such group is involved in the formation of semicarbazones. Why? (3)
Answer:
Due to resonance one – NH2 group undergoes or involved in resonance and hence can’t participate in the formation of semicarbazone. Long pair of – NH2 group is not involved in resonance and is available for nucleophillic attack.
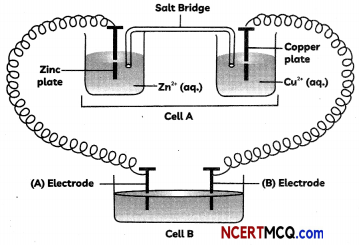
Question 5.
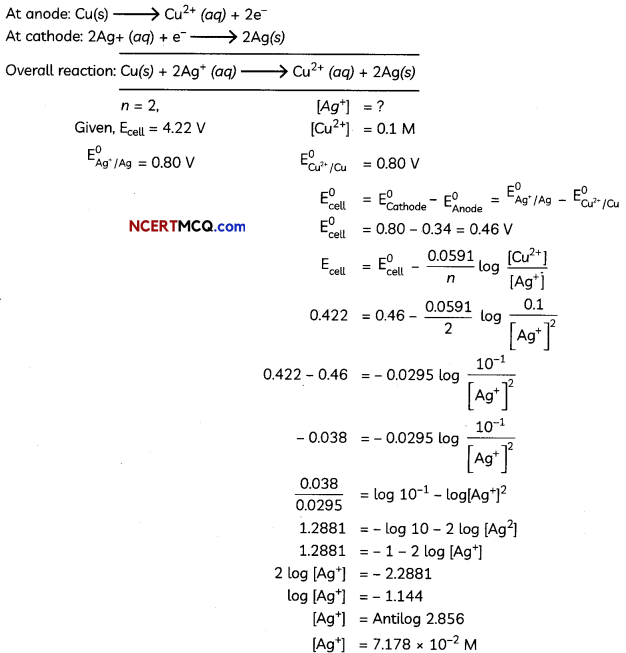
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured 0.422 V. Determine the concentration of silver ion in the celL
Given : E°Ag+/Ag = + 0.80 V, E°Cu2+/cu = + 0.34 V.
OR
A current was passed for 5 hours through two electrolytic cells connected in series. The first cell contains AuCl3 and second cell CuSO4 solution. If 9.85 g of gold was deposited in the first cell, what amount of copper gets deposited in the second cell? Also calculate magnitude of current in ampere.
Given: Atomic mass of Au = 197 amu and Cu = 63.5 amu (3)
Answer:
Related Theory:
Students should not forget to put powers of stoichiometric coefficients on the concentrations of products and reactants in Nernst equation.
OR
According to Faraday’s second law
Equivalent of gold formed = Equivalent of Cu formed
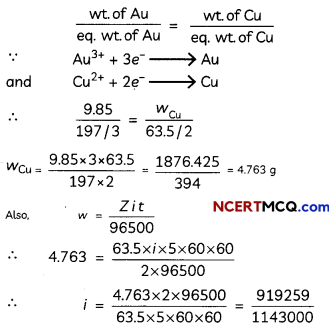
= 0.804 A
Related Theory:
Students often forget to divide Molar mass by F (Faraday) along with n (no. of electrons) for the calculation of Z (gram equivalent).
![]()
Question 6.
Give reasons for the following:
(A) pKb value for aniline is more than that for methylamine.
(B) Ethylamine is soluble in water whereas aniline is not soluble in water.
(C) Primary amines have higher boiling points than tertiary amines.
OR
Three test tubes labelled as A, B and C contain three types of amines i.e., primary, secondary and tertiary. Describe a method which can be used for the identification of the amines. Also write chemical equations of the reaction involved. 3
Answer:
(A) Aniline undergoes resonance and as a result the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate whereas in methylamine (due to the +1 effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
Explanation:
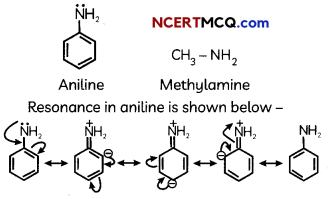
(B) Ethylamine when added to water forms intermolecular H—bonds with water. Hence, it is soluble in water but aniline can form H-bonding with water to a very small extent due to the presence of a large hydrophobic -C6H5 group. Hence. aniline is insoluble in water.
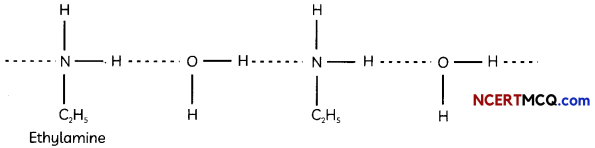
(C) The extent of inter-molecular hydrogen bonding in primary amines is higher than the secondary amines. Secondary amine has only one hydrogen, and tertiary amines have no hydrogen atom for inter-molecular hydrogen bonding whereas primary amine has two hydrogen atom available for inter-molecular hydrogen bonding.
Explanation: Higher the extent of hydrogen bonding, higher ¡s the boiling point.
![]()
OR
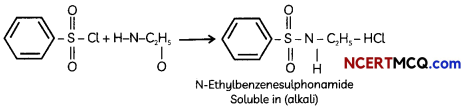
Hinsberg test is used identication of primary. secondary ad tertiary amine. Benzenesulphonyl chloride (C6H5SO2Cl). which is also known as Hinsbergs reagent, reacts with primary and secondary amines to form sulphonamides.
The reaction of benzenesulphonyl chloride with primary amine yields N-ethylbenzenesulphonyl amide. The hydrogen attached to nitrogen in sulphonomide is strongly acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is soluble in alkali.
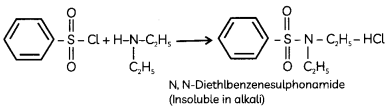
In the reaction with secondary amine, N, N- diethylbenzenesulphonamide is formed. Since N, N-diethylbenzene sulphonamide does not contain any hydrogen atom attached to nitrogen atom, it is not acidic and hence insoluble in alkali.
Tertiary amines do not react with benzenesulphonyl chloride due to the absence of replaceable hydrogen.
Question 7.
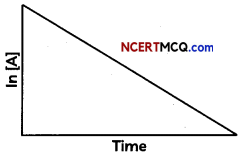
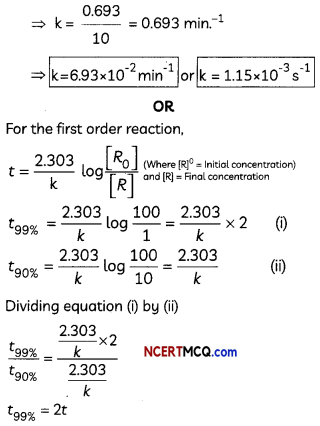
Show that In case of first order reaction, the time required for 99.9% completion is nearly ten times the time required for half completion of the reaction. (3)
Answer:
For first order reaction.
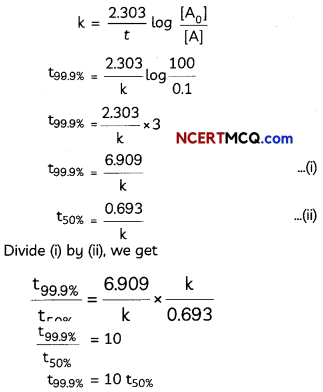
![]()
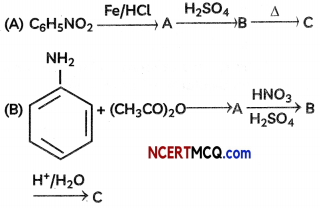
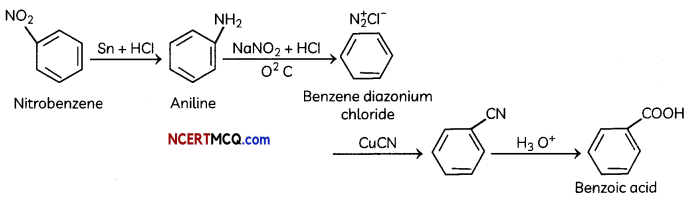
Question 8.
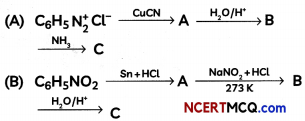
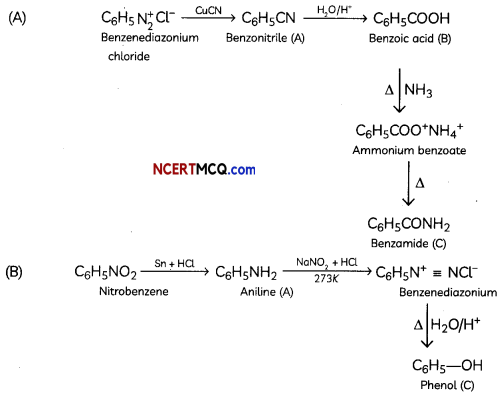
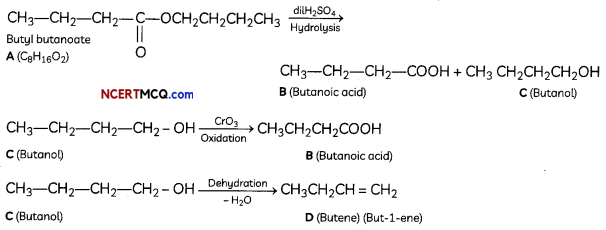
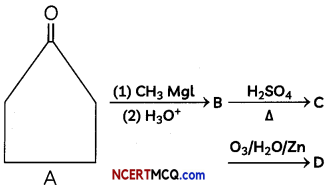
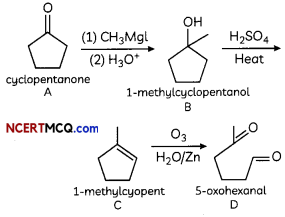
An organic compound with molecular formula C9H10O forms 2, 4, – DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation it gives 1, 2-benzene-di- carboxylic acid. Identify the compound.
OR
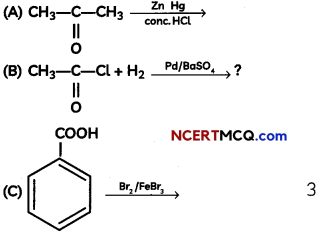
Answer:
It is on aLdehyde or ketone os it forms 2,4-DNP derivative.
As the compound reduces Tollen’s reagent and undergoes cannizzaro reaction, it is an aldehyde and not a ketone.
On vigorous oxidation, it gives 1, 2-benzenedicarboxylic acid. So. it must have an aLkyL group at o-ortho position with respect to CHO group on the benzene ring.
Motecular formula suggests that it should be 2-ethyl benzoldehyde.
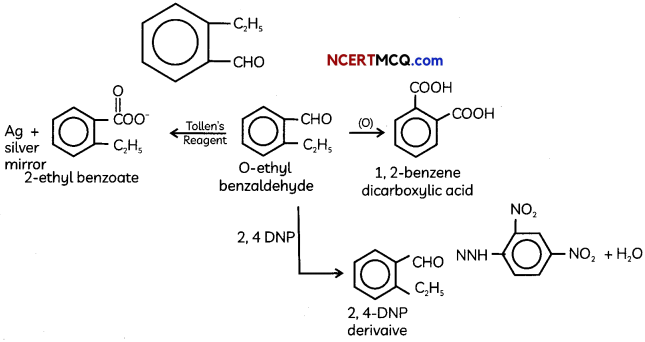
OR
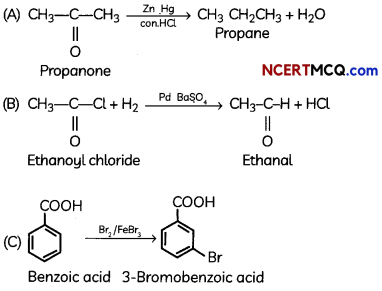
![]()
Question 9.

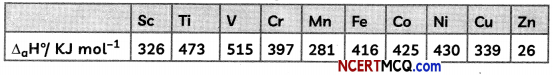
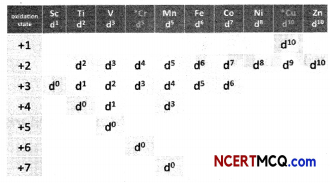
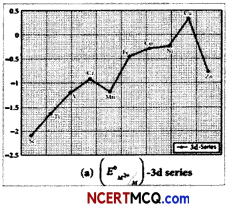
Observe the table and give reasons for the following trends
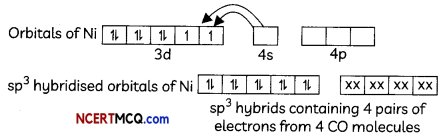
(A) The second ionization enthalpy vaLues of Cr and Cu are unusually high.
Answer:
Due to extra stability of half filled d orbitals in Cr+ (3d5) and full filled orbitals in Cu+ (3d10), second ionization enthalpies for Cr and Cu are very high.
(B) The second Ionization enthalpy of Zn is comparatively low.
Answer:
Second electron in Zn+ is to be removed from 4s1 because it is very less stable so second ionization enthalpy of Zn is low.
(C) The third ionization enthalpy of Mn and Zn are unsually high.
Answer:
Electronic configuration of Mn2+ is [Ar]3d10 4s0 and of Zn2+ is [Ar]3d10 4s0. Due to extra stability of half filled and full filled orbitals, third ionization enthalpies for both the elements are very high.
![]()
Question 10.
Explain the following terms with help of one example of each: (3)
(A) Ambidentate ligands
Answer:
Ambidentate ligands: The monodentate ligands which can coordinate with the central atom through more than one site are called ambidentate ligands.
These ligands contain more than one coordinating atoms in their molecules.
For example, NO2 can coordinate to the metal atom through N or O as follows –
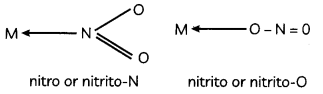
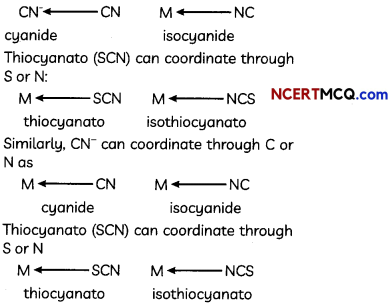
Explanation: Other examples are as follows: coordinate through C or N as
(B) Spectra chemical series
Answer:
Spectrochemical series: The arrangement of Ligands in the increasing order of crystal field splitting is called spectrochemical series. It is an experimentally determined series based on the absorption of light by complexes with different ligands. This is shown below.
I– < Br– < SCN– < Cl– < F– < OH– < Ox2- < O2- < H2O < NCS– < Py = NH3 2- < CN– < CO.
Related Theory:
Weak Held ligands are those which cause less crystal field splitting. These form high spin complexes. For example, Cl–, F–, etc.
Strong field ligands are those which cause greater crystal field splitting. These form low spin complexes. For example, CN–, NO2–, CO.
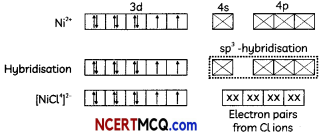
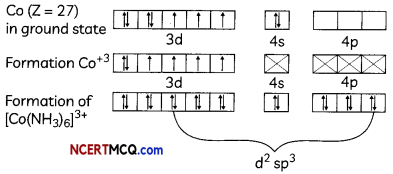
(C) Heteroleptic complexes
Answer:
Heteroleptic complexes: The complexes in which the metal is bound to more than one kind of donor groups (ligands) are called heteroleptic complexes. Some common examples of heteroleptic complexes are [NiCl2(H2O)4], [CoCl2(NH3)4]+, etc
![]()
Question 11.
(A) Describe the general trends in the following properties of the first series (3d) of the transition elements:
(i) Number of oxidation states exhibited
(ii) Formation of oxometal ions
(B) The transition metals and many of their compounds act as good catalysts. Give reason.
OR
(A) Assign reasons for the following:
(i) Copper(l) ion is not known to exist in aqueous solutions.
(ii) Both O2 and F2 stabilize high oxidation states of transition metals but the ability of oxygen to do so exceeds that of fluorine.
(B) Which of the following cations are coloured in aqueous solutions and why?
Sc3+, V3+, Ti4+, Mn2+
(At. Nos. Sc = 21, V = 23, Ti = 22, Mn = 25) (3)
Answer:
(A) (i) The number of oxidation states increases upto middle of series i.e. unto +7 and then decreases.
Explanation: The numbers of the oxidation states increase on moving from Sc to Mn. On moving from Mn to Zn, the number of the oxidation states decrease due to a decrease in the number of available unpaired electrons. The relative stability of the +2 oxidation state increases on moving from top to bottom. This is because on moving from top to bottom, it becomes more and more difficult to remove the third electron from the d orbital.
(ii) Oxometal ions are polyatomic ions with oxygen. Example: VO2+, VO2+, TiO2+
Explanation: The elements of the first series of transition metals form a variety of oxides of different oxidation states having general formulae MO, M2O3, M3O6, MO2, MO3, etc. All metals except Sc form MO oxides which are ionic in nature. Beyond group 7, no higher oxides except Fe2O3 are known. The oxocations stabilise as VO2+, VO2+ and TiO2+.
(B) Transition metals and their compounds act as good catalysts due to its ability to show variable oxidation state and form complexes and they provide a suitable surface for reaction to take place.
OR
(A) (i) Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionization enthalpy of copper is large but Ahyd (hydration enthalpy) for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it more than compensates for the second ionization enthalpy of copper. Therefore, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation as follows:
2Cu+ → Cu2+ + Cu
(ii) The ability of O2 to stabilize higher oxidation states exceeds that of fluorine because oxygen can form multiple bonds with metals.
(B) V3+ and Mn2+ are coloured, due to the presence of unpaired (d3 in V3+ and d5 in Mn2+) electrons, they can undergo d-d transitions. Others are colourless due to the absence of unpaired electrons and cannot undergo d-d transitions.
Explanation: The absorption of visible light and hence coloured nature of the transition metal cations is due to the promotion of one or more unpaired d-electron from a lower to a higher level within the same d-subshell. This promotion requires small amount of energy available in the visible light. This is called d-d transition.
![]()
Section – C
(Section C-Question No 12 is case-based question carrying 5 marks.)
Question 12.
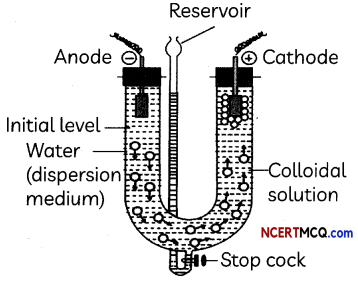
Read the passage given below and answer the questions that follow:
Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. In a sense, they bridge the microscopic and the macroscopic. As such, they possess some of the properties of both, which makes colloidal matter highly adaptable to specific uses and functions. Colloid science is central to biology, food science and numerous consumer products.
Colloidal dispersions appear to be homogeneous, and the colloidal particles they contain are small enough (generally between 1-1000 nm) to exhibit Brownian motion, cannot be separated by filtration, and do not readily settle out. But these dispersions are inherently unstable and under certain circumstances, most colloidal dispersions can be “broken” and will “flocculate” or settle out.
Particles composed of ionic or ionizable substances usually have surface charges due to adsorption of an ion (usually an anion) from the solution, or to selective loss of one kinds of ion from the crystal surface. For example, Ag+ ions on the surface of a silver iodide crystal go into solution more readily than the Br~ ions, leaving a negatively – charged surface.
Charged colloidal particles will attract an excess of oppositely – charged counter-ions to their vicinity from the bulk solution, forming a localized “cloud” of compensating charge around each particle. The entire assembly is called an electric double layer. Electric double layers of one kind or another exist at all phase boundaries, but those associated with colloids are especially important
(A) What is the size of colloidal particles?
(B) Name the property of colloidal solutions due to which their particles do not settle down.
(C) What is the cause of charge on colloidal particles?
(D) How is the electrical double layer formed in colloidal solutions? What is the other name given to the double layer?
OR
How is electrokinetic potential is produced between the two layers of charges? Write the term used for this potential. (5 )
Answer:
(A) Size of colloidal particles lies between the size of particle of true solutions and suspensions and their particles size range is generally between 1-1000 nm.
(B) Brownian movement
Explanation: Colloidal particles in a sol are continuously bombarded by the molecules of the dispersion medium on all sides. As a result the sol particles show random or zig-zag movements. This random or zig¬zag motion of the colloidal particles in a sol is called Brownian motion or Brownian movement
(C) The charge on the colloidal particles is due to adsorption of common ions of the electrolyte on the surface of the colloidal particles, e.g., Fe3+ from FeCl3 on the surface of Fe(OH)3 particles.
(D) One type of the ions (common ion) of the electrolyte are adsorbed on the surface of colloidal particles due to preferential adsorption, it forms a “Fixed layer”. It attracts the opposite ions to form another layer called “diffused layer”.
The double layer of opposite charge thus formed is called Helmholtz electrical double layer.
OR
As the separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layer result in a difference in potential between these layers.
This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.

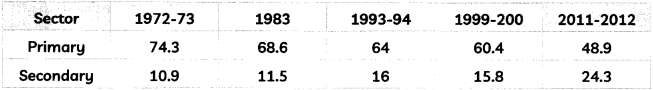
 Read the following text carefully and answer question number 8 and 9 given below:
Read the following text carefully and answer question number 8 and 9 given below: