Check the below NCERT MCQ Questions for Class 12 Chemistry Chapter 13 Amines with Answers Pdf free download. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest exam pattern. We have provided Amines Class 12 Chemistry MCQs Questions with Answers to help students understand the concept very well.
Class 12 Chemistry Chapter 13 MCQ With Answers
Chemistry Class 12 Chapter 13 MCQs On Amines
Amines MCQ Class 12 Chapter 13 Question 1.
Nitrogen atom of amino group is ………. hybridised.
(a) sp
(b) sp2
(c) sp3
(d) sp3d
Answer
Answer: (c) sp3
Amines MCQ Pdf Download Class 12 Chapter 13 Question 2.
Which of the following should be most volatile?
I. CH3CH2CH2NH2
II. (CH3)3N
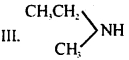
IV. CH3CH2CH3
(a) II
(b) IV
(c) I
(d) III
Answer
Answer: (b) IV
MCQ On Amines Chapter 13 Question 3.
C3H8N cannot represent
(a) 1° ammine
(b) 2° ammine
(c) 3° ammine
(d) quartemary ammonium salt
Answer
Answer: (d) quartemary ammonium salt
MCQ On Amines Class 12 Chapter 13 Question 4.
Identify the correct IUPAC name
(a) (CH3CH2)2NCH3 = N-Ethyl-N-methylethanamine
(b) (CH3)3CNH2 = 2-methylpropan-2-amine
(c) CH3NHCH (CH3)2 = N-Methylpropan-2-amine
(d) (CH3)2CHNH2 = 2, 2-Dimethyl-N-propanamine
Answer
Answer: (a) (CH3CH2)2NCH3 = N-Ethyl-N-methylethanamine
Amines Class 12 MCQ Chapter 13 Question 5.
The most convenient method to prepare primary (i Amine) amine containing one carbon atom less is
(a) Gabriel phthalmidie synthesis
(b) Reductive amination of aldehydes
(c) Hofmann bromamide reaction
(d) Reduction of isonitriles
Answer
Answer: (c) Hofmann bromamide reaction
Amines MCQ Pdf Class 12 Chapter 13 Question 6.
Identify the correct pathway to convert propanoic acid to ethylamine. The reagent represented by A, B and C are
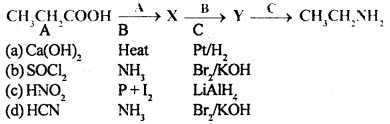
Answer
Answer: (b)
MCQ Of Amines Class 12 Chapter 13 Question 7.
When excess of ethyl iodide is treated with ammonia, the product is
(a) ethylamine
(b) diethylamine
(c) triethylamine
(d) tetrathylammonium iodide
Answer
Answer: (d) tetrathylammonium iodide
Amines Class 12 MCQ Pdf Chapter 13 Question 8.
Amides may be converted into amines by a reaction named after
(a) Hofmann Bromide
(b) Claisen
(c) Perkin
(d) Kekule
Answer
Answer: (a) Hofmann Bromide
MCQ On Amines Pdf Chapter 13 Class 12 Question 9.
Reduction of CH3CH2NC with hydrogen in presence of Ni or Pt as catalvst gives
(a) CH3CH2NH2
(b) CH3CH2NHCH3
(c) CH3CH2NHCH2CH3
(d) (CH3)3N
Answer
Answer: (b) CH3CH2NHCH3
Aniline Is In Nature MCQ Chapter 13 Class 12 Question 10.
Secondary amines can be prepared by
(a) reduction of nitro compounds
(b) oxidation of N-substituted amides
(c) reduction of isonitriles
(d) reduction of nitriles
Answer
Answer: (c) reduction of isonitriles

Amines MCQ Questions Chapter 13 Class 12 Question 11.
Which of the following amides will give ethylamine on reaction with sodium hypobromide?
(a) Butanamide
(b) Propanamide
(c) Acetamide
(d)Benzamide
Answer
Answer: (b) Propanamide
MCQ On Aromatic Amines Chapter 13 Class 12 Question 12.
Benzoic acid is treated with SOCl2 and the product (X) formed is reacted with ammonia to give (Y). (Y) on reaction with Br2 and KOH gives (Z). (Z) in the reaction is
(a) aniline
(b) chlorobenzene
(c) benzamide
(d) benzoyl chloride
Answer
Answer: (a) aniline
MCQ Questions On Amines Chapter 13 Class 12 Question 13.
Which one of the following reducing agents is likely to be most effective in bringing about the following change?
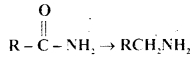
(a) H2-Ni
(b) NaBH4
(c) LiAlH4 ether
(d) Na-AIcohol
Answer
Answer: (c) LiAlH4 ether
MCQ On Basicity Of Amines Chapter 13 Class 12 Question 14.
Amine that cannot be prepared by Gabricl-Phthalmidie synthesis is
(a) aniline
(b) benzyl amine
(c) methyl amine
(d) iso-butylamine
Answer
Answer: (a) aniline
MCQs Based On Amines Chapter 13 Class 12 Question 15.
What is the end product in the following sequence of reactions?
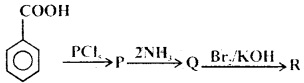
(a) Aniline
(b) Phenol
(c) Benzene
(d) Benzenediazxonium chloride
Answer
Answer: (a) Aniline
Question 16.
Tertiary amines have lowest boiling points amongst isomeric amines because
(a) they have highest molecular mass
(b) they do not form hydrogen bonds
(c) they are more polar in nature
(d) they are most basic in nature
Answer
Answer: (b) they do not form hydrogen bonds
Question 17.
Which of the following amines will give carbylatnine reaction?
(a) (C2H5)3N
(b) (C2H5)2NH
(c) C2H5NH2
(d) C3H7NHC2H35
Answer
Answer: (c) C2H5NH2
Question 18.
![]()
in the above reaction is
(a) CH3CH2CH2NHCOCH3
(b) CH3CH2CH2NH2
(c) CH3CH2CH2CONHCH3
(d) CH3CH2CH2CONHCOCH3
Answer
Answer: (a) CH3CH2CH2NHCOCH3
Question 19.
The end product Z of the reaction
![]()
(a) propanenitrile
(b) triethylamine
(c) diethylamine
(d) propylamine
Answer
Answer: (a) propanenitrile
Question 20.
Primary and secondary amines are distinguished by
(a) Br2/ROH
(b) HClO
(c) HNO2
(d) NH3
Answer
Answer: (c) HNO2
Question 21.
Arrange the following compounds in increasing order of basicity:
CH3NH2, (CH3)2 NH, NH3, C6H5NH2
(a) C6H5NH2 < NH3 < (CH3)2NH < CH3NH2
(b) CH3NH2 < (CH3)2NH < NH3 < C6H5NH2
(c) C6H5NH2 <NH3 < CH3NH2<(CH3)2NH
(d) (CH3)2NH < CH3NH2 <NH3 < C6H5NH2
Answer
Answer: (c) C6H5NH2 <NH3 < CH3NH2<(CH3)2NH
Question 22.
Which of the following species are involved in the carbvlamine test?
(i) RNC
(ii) CHCl3
(iii) COCl2
(iv) NaNO2 + HCl
(a) (i) and (iv)
(b) (i) and (ii)
(c) (ii) and (iv)
(d) (ii) and (iii)
Answer
Answer: (b) (i) and (ii)
Question 23.
Identify ‘Z’ in the sequence?
![]()
(a) C6H5CN
(b) C6H5CONH2
(c) C6H5COOH
(d) C6H5CH2NH2
Answer
Answer: (c) C6H5COOH
Question 24.
Which of the following from isocyanide on reaction with CHCl and KOH?
(a) C6H5NHCH3
(b) CH3C6H4NH2
(c) C6H5NHC4H9.
(d) C6H5N (C2H5)2
Answer
Answer: (b) CH3C6H4NH2
Question 25.
Which of the following is used as Hinsberg’s reagent?
(a) C6H5SO2Cl
(b) C6H5SO3H
(c) C6H5NHCH3
(d) C6H5COCH3
Answer
Answer: (a) C6H5SO2Cl
Question 26.
Electrophilic substitution of aniline with bromine water at room temperature gives
(a) 2-bromoaniline
(b) 3-bromoaniline
(c) 2, 4, 6-tribromoaniline
(d) 3, 5, 6-tribromoaniline
Answer
Answer: (c) 2, 4, 6-tribromoaniline
Question 27.
Among the compounds C3H7NH2, CH3NH2, C2H5NH2, and C6H5NH2. Which is the least basic compound?
(a) CH3NH2
(b) C2H5NH2
(c) C3H7NH2
(d) C6H5NH2
Answer
Answer: (d) C6H5NH2
Question 28.
Anilinium hydrogensulphate on heating with sulphuric acid at 453-473 K produces
(a) sulphanilic acia
(b) benzenesulphonic acid
(c) aniline
(d) anthranilic acid
Answer
Answer: (a) sulphanilic acia
Question 29.
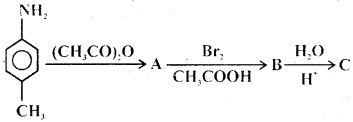
Product would be
Answer
Answer: (c)
Question 30.
Which of the following can exist as zwitter ion?
(a) p-Aminoacetophenone
(b) Sulphanilic acid
(c) p-Nitroaminobenzene
(d) p-Methoxyphenol
Answer
Answer: (b) Sulphanilic acid
Question 31.
Reduction of aromatic nitro-compounds using Sn and HCl gives
(a) aromatic primary amines
(b) aromatic secondary amines
(c) aromatic tertiary amines
(d) aromatic amides
Answer
Answer: (a) aromatic primary amines
Question 32.
Which amine amongst the following will answer positively the carbylamine test (i.e., heating with CHCl3 and KOH)?
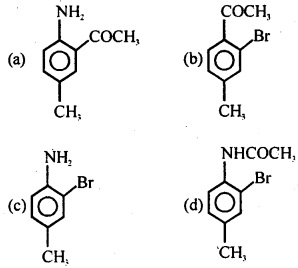
(a) C6H5 – NH – CH3
![]()
(c) C6H5 – NH – C4H9
(d) C6H5 -N(C2H5)3
Answer
Answer: (b)
Question 33.
Most basic species amongst the following is
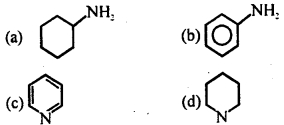
Answer
Answer: (d)
Question 34.
When aniline is heated with cone. H2SO4 at 455-475 K, it forms
(a) aniline hydrogensulphate
(b) sulphanilic acid
(c) amino benzene sulphonic acid
(d) benzenesulphonic acid
Answer
Answer: (b) sulphanilic acid
Question 35.
Which of the following compounds cannot be identified by carbylamine test?
(a) CH3CH2NH2
(b) (CH3)2CHNH2
(c) C6H5NH2
(d) C6H5NHC6H5
Answer
Answer: (d) C6H5NHC6H5
Question 36.
What is obtained when benzoyl chloride reacts with aniline in the presence of sodium hydroxide?
(a) Benzoic acid
(b) Benzanilide
(c) Acetanilide
(d) Azobenzene
Answer
Answer: (b) Benzanilide
Question 37.
Which of the following compounds reacts with NaNO2 and HCl at 0-4°C to give alcohol/phenol?
(a) C6H5NH2
(b) C2H5NH2
(c) CH3NHCH3
(d) C6H5NHCH3
Answer
Answer: (b) C2H5NH2
Question 38.
Which of the following has highest pKb value?
(a) (CH3)3CNH2
(b) NH3
(c) (CH3)2MH
(d) CH3NH3
Answer
Answer: (b) NH3
Question 39.
Acetylation of a secondary amine in alkaline medium yields
(a) N, N-dialkyl acetamide
(b) N, N-dialkyl amine
(c) N, N-dialkyl amide
(d) acetyl dialkyl amine
Answer
Answer: (a) N, N-dialkyl acetamide
Question 40.
Which of the following is amphoteric in nature?
(a) CH3NH2
(b) CH3NHCH3
(c) CH3CONH2
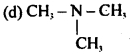
Answer
Answer: (c) CH3CONH2
Question 41.
The amines are basic in nature, hence they form salts with hydrochloric acid. Which of the following will be insoluble in dil. HCl?
(a) C6H5NH3
(b) (C6H5)3N
(c) C2H5NH2
(d) CH3NHCH3
Answer
Answer: (b) (C6H5)3N
Question 42.
Primary, secondary and tertiary amines may be separated by using
(a) iodoform
(b) diethyloxalate
(c) benzene sulphonyl chloride
(d) acetyl chloride
Answer
Answer: (c) benzene sulphonyl chloride
Question 43.
When p-toluidine reacts with chloroform and alcoholic KOH, then the product is
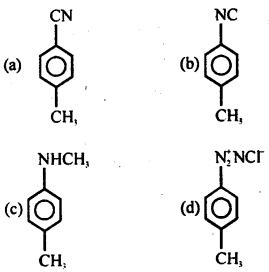
Answer
Answer: (b)
Question 44.
An organic compound (X) was treated with sodium nitrite and HCl in ice cold conditions. Bubbles of nitrogen gas were seen coming out. The compound (X) may be
(a) a secondary aliphatic amine.
(b) a primary aromatic amine
(c) a primary aliphatic amine
(d) a tertiary amine
Answer
Answer: (c) a primary aliphatic amine

Question 45.
Among the following:
I. CH3NH5
II. (CH3)2NH
III. (CH3)3N
IV. C6H5NH2
Which will give the positive carbylamine test?
(a) I and II
(b) I and IV
(c) II and IV
(d) II and III
Answer
Answer: (b) I and IV
Question 46.
Cyclohexyiamine is stronger base than aniline become
(a) in aniline electron pair is involved in conjugation
(b) in cyclohexyiamine electron pair is involve conjugation
(c) in aniline-NH, group is protonated
(d) in cyclohexyiamine nitrogen has a negative charge
Answer
Answer: (a) in aniline electron pair is involved in conjugation
Question 47.
The strongest base among the following is
(a) C6H5NH3
(b) p-NH2C6H4NH
(c) m-NO2C6H4NH2
(d) C6H5CH2NH2
Answer
Answer: (d) C6H5CH2NH2
We hope the given NCERT MCQ Questions for Class 12 Chemistry Chapter 13 Amines with Answers Pdf free download will help you. If you have any queries regarding Amines CBSE Class 12 Chemistry MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 12 Chemistry MCQ:
- The Solid State Class 12 MCQ
- Solutions Class 12 MCQ
- Electrochemistry Class 12 MCQ
- Chemical Kinetics Class 12 MCQ
- Surface Chemistry Class 12 MCQ
- General Principles and Processes of Isolation of Elements Class 12 MCQ
- The p-Block Elements Class 12 MCQ
- The d-and f-Block Elements Class 12 MCQ
- Coordination Compounds Class 12 MCQ
- Haloalkanes and Haloarenes Class 12 MCQ
- Alcohols, Phenols and Ethers Class 12 MCQ
- Aldehydes, Ketones and Carboxylic Acids Class 12 MCQ
- Amines Class 12 MCQ
- Biomolecules Class 12 MCQ
- Polymers Class 12 MCQ
- Chemistry in Everyday Life Class 12 MCQ
