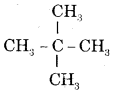
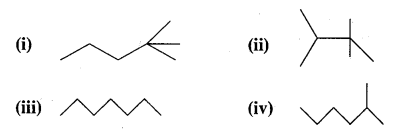
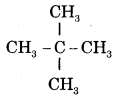
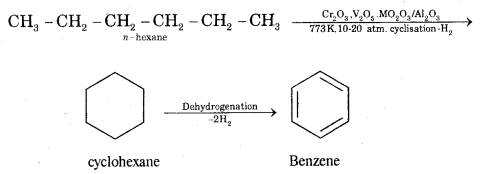
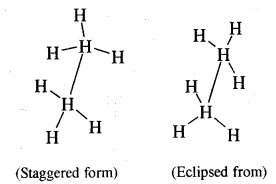
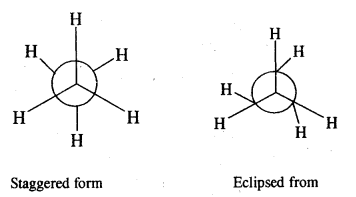
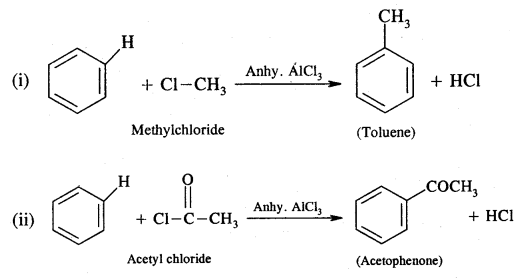
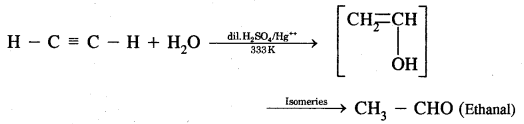
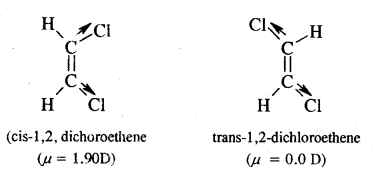
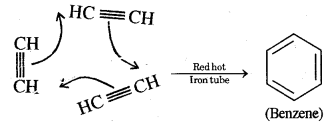
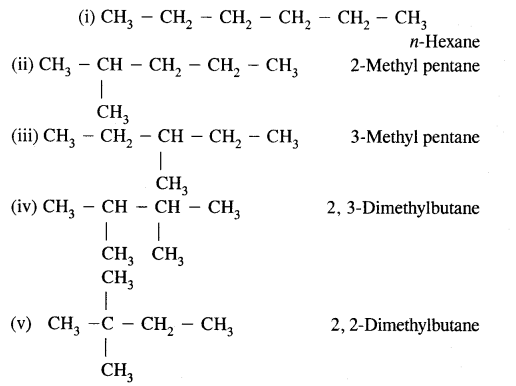
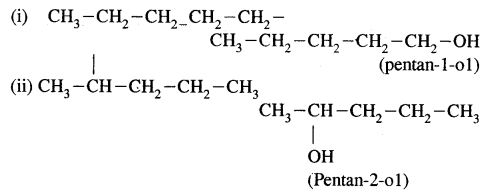
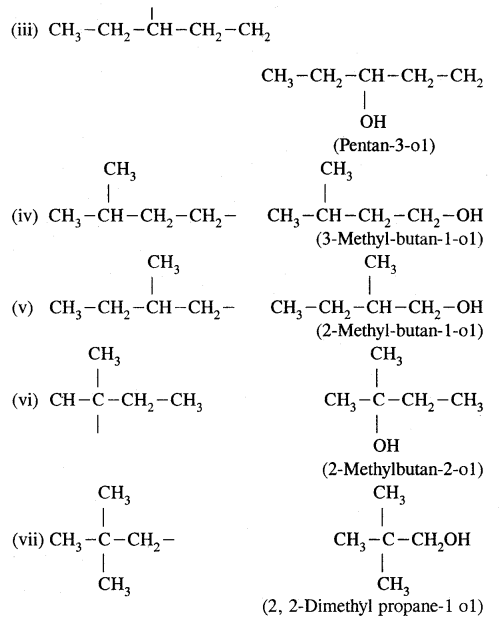
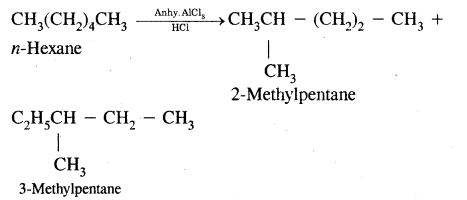
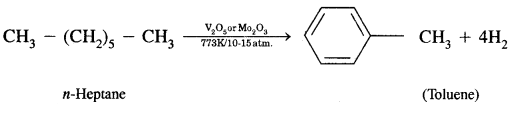
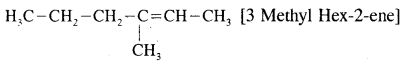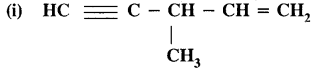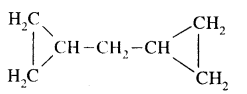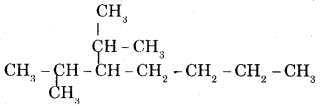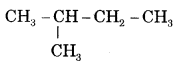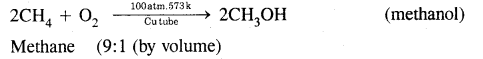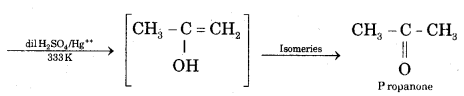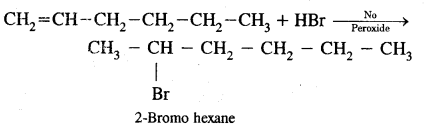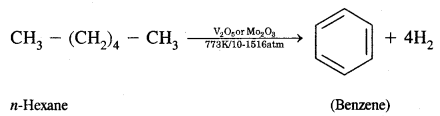CA Foundation Business & Commercial Knowledge Study Material Chapter 2 Business Environment – Test Questions
CA Foundation Business and Commercial Knowledge Study Material Chapter 2 Business Environment – Test Questions
1. Which of the following is a characteristic of business environment?
(a) Aggregative
(b) Dynamic
(c) Uncertain
(d) All of them.
2. Which of the following is an element of micro environment:
(a) Customers
(b) Competitors
(c) Suppliers
(d) All of them.
3. Which of following relates to population?
(a) Demographic environment
(b) Social environment
(c) Cultural environment
(d) Natural environment
4. Understanding of environment enables a business enterprise to
(a) focus on customers
(b) gain the first mover advantage
(c) become aware of impending threat
(d) All of them.
5. Demonetization and GST are examples of changes in
(a) Political environment
(b) Social environment
(c) Technological environment
(d) Global environment
6. Merger of associate banks of SBI into SBI is an example of changes in
(a) Economic environment
(b) Technological environment
(c) Social environment
(d) Demographic environment
7. Tick (✓) the correct alternative.
Demographic trends are a part of:
(a) economic environment III
(b) social environment
(c) political environment
(d) legal environment
8. State whether the following statements are True or False:
Wait and watch is a response of least resistance.
Big and powerful firms adopt the response of gaining command over the environment.
Innovative approach requires no feedback system.
Adaptation response involves anticipation of changes in business environment.
9. State whether the following statements are true or false.
Privatisation and globalisation are components of economic liberalisation.
Pressures for structural adjustments are a reason for globalisation.
Denationalisation is a form of privatisation.
Closure of small scale firms is a positive effect of economic liberalisation.
10. Fill in the blanks:
- Business environment is the totality of …………… forces
- Different elements of business environment are ……………
- When business environment changes rapidly and suddenly …………… increases.
- Industrial policy, monetary policy and fiscal policy are elements of …………… environment of business.
- Business gets …………… from the environment and supplies …………… to the environment.
11. Match the items in column A with those in column B

12. Age, family size, sex composition and other people related elements are part of
(a) political environment
(b) economic environment
(c) demographic environment
(d) natural environment.