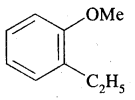
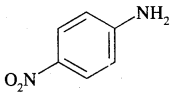
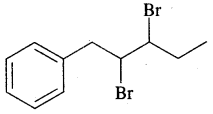
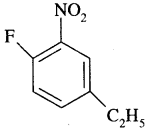
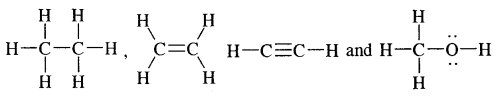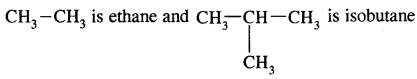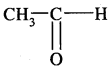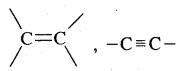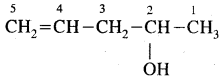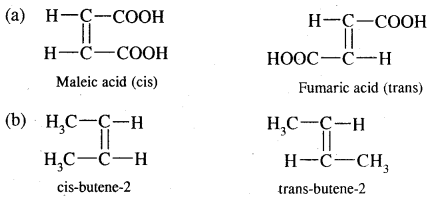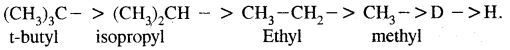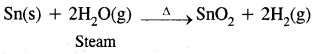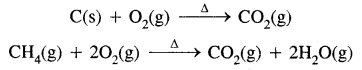By going through these CBSE Class 11 Chemistry Notes Chapter 13 Hydrocarbons, students can recall all the concepts quickly.
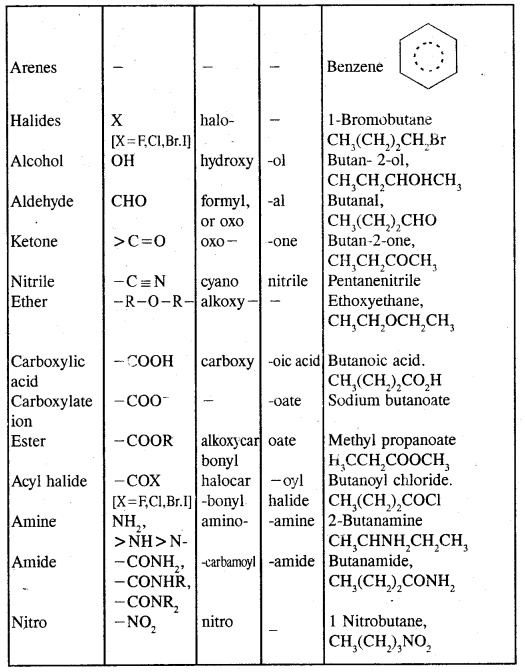
Hydrocarbons Notes Class 11 Chemistry Chapter 13
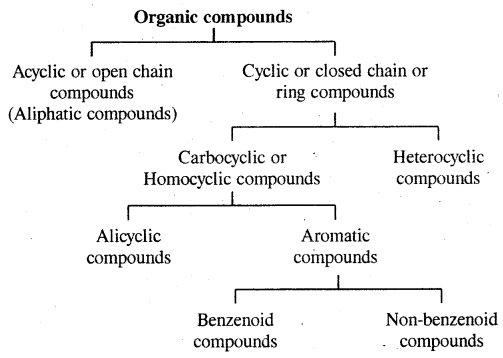
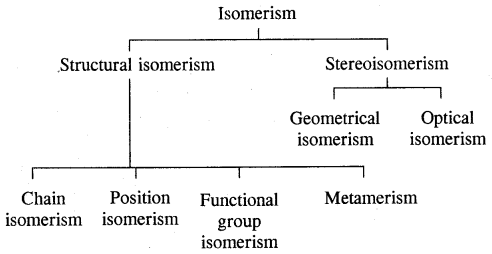
→ Classification-classification of hydrocarbons.
→ Alkanes-Nomenclature. isomerism, preparation, properties of alkanes, conformations.
→ Alkenes-structure of double bonds, Nomenclature, Isomerism, preparation and properties.
→ Alkynes-Nomenclature of isomerism, the structure of the triple bond, preparation & properties.
→ Aromatic hydrocarbons-Nomenclature & isomerism structure of benzene, Aromaticity, preparation & properties.
→ Directive influence of a functional group in mono-substituted benzene.
→ Carcinogenicity & Toxicity Benzene of polynuclear hydrocarbons.
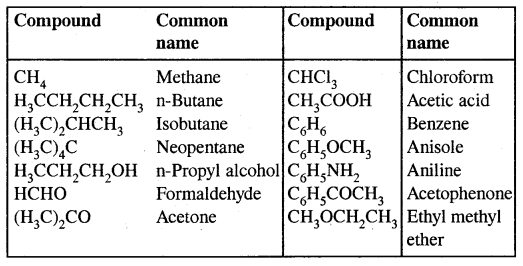
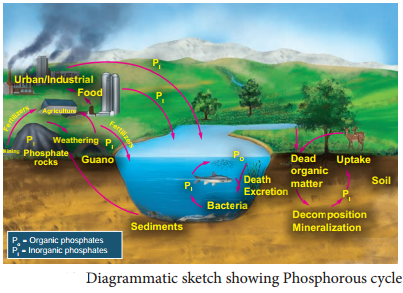
→ Hydrocarbons: Hydrocarbons are the compounds of carbon & hydrogen only. Hydrocarbons are mainly obtained from coal & petroleum.
→ Petrochemical: Petrochemicals are the prominent starting material used for the manufacture of a large number of commercially important products.
→ L.P.G.: Liquified petroleum gas
→ C.N.G.: Compressed natural gas.
→ Classification of hydrocarbons: Saturated, unsaturated, cyclic (alicyclic) & Aromatic
→ Important reactions of Alkanes: Free radical substitution, combustion, oxidation & aromatization.
→ Alkenes & Alkynes: Undergo mainly addition reactions, (electrophilic additions).
→ Aromatic hydrocarbons: Despite having unsaturation undergo mainly electrophilic substitution reactions
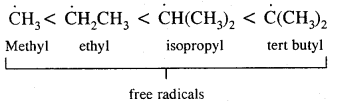
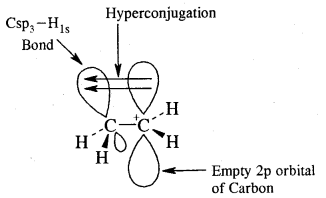
→ Conformation Isomerism: Alkanes show conformational isomerism due to free rotation along with the C – C sigma bonds. Out of staggered of the eclipsed conformations of ethane, staggered conformations are more stable as hydrogen atoms are farthest apart.
→ Geometrical Isomerism: Alkanes exhibits geometrical isomerism (cis-trans) due to restricted rotation around the carbon-carbon double bond
→ Huckel Rule: Benzene of benzenoid compounds show aromatic character. Aromaticity, the property of being aromatic is possessed by compounds having specific electronic structure characterized by Huckel Rule (4n + 2) π electron rule.
→ Carcinogenic property: Some of the polynuclear hydrocarbons having fused benzene ring system have carcinogenic property.
→ Activation & deactivation of benzene ring: The nature of groups or substituents attached to the benzene ring is responsible for activation or deactivation of the benzene ring towards further electrophilic substitution and also for orientation of the incoming group.
Friedel-crafts Reaction:
1. Friedel craft alkylation reaction
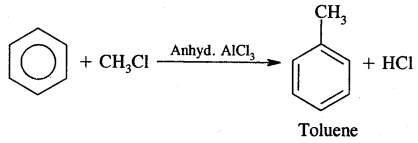
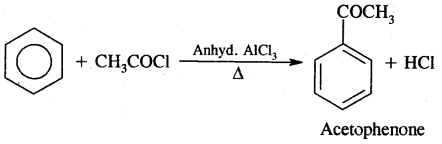
2. Friedel craft acylation reaction
Structure of Benzene
Kekule’s Structure
→ Markownikov Rule: The rule states that the negative part of the addendum gets attached to that carbon atom which possesses a lesser number of hydrogen atoms as:
CH3 – CH = CH2 + HBr →
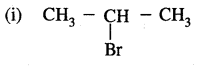
2-Bromopropane (Main product)
(ii) CH3 CH2 CH2Br
1-Bromopropane (Minor product)
→ Lindlar’s Catalyst: Partially deactivated palletised charcoal is known as Lindlar’s Catalyst.
Chapter In Brief:
Hydrocarbons are the compounds of carbon and hydrogen only, Alkanes, Alkenes, alkynes, and aromatic compounds constitute hydrocarbons. Alkanes are saturated hydrocarbons containing carbon-carbon single bonds. Alkenes are unsaturated hydrocarbons containing at least one
double bonds, whereas alkynes are unsaturated hydrocarbons containing at least one — C ≡ C — triple bond.
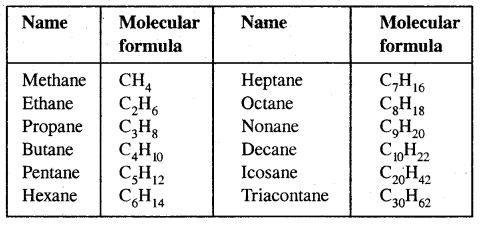
Alkanes: Earlier known as paraffin, the general formula of their homologous series is CnH2n+2.
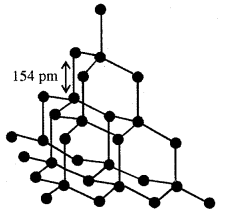
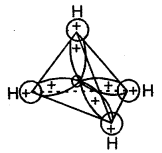
Methane, the first member is having a tetrahedral shape according to VSEPR Theory. It is multiplanar in which a carbon atom lies at the centre and four hydrogen atoms lie at the four corners of a regular tetrahedron. H-C-H bond angle is 109.5°. In alkenes, C-C and C-H bond lengths are 154 pm and 112 pm respectively. C-C and C – H bonds are formed by head-on the overlapping of sp3 hybrid orbitals of carbon and Is atomic orbitals of hydrogen atoms.
Nomenclature & Isomerism in Alkanes:
The first three members of the alkane family namely methane, ethane and propane have only one structure but higher alkanes can have more than one structure.
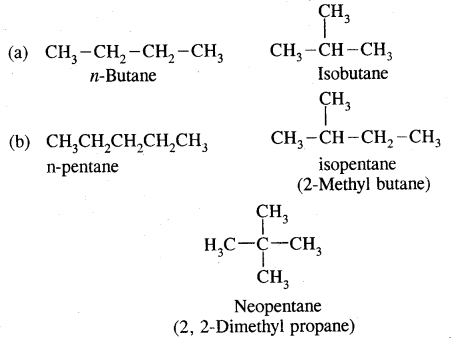
e.g. C4H10 have the following two structures
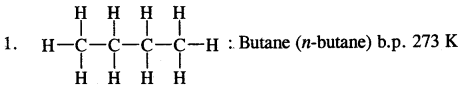
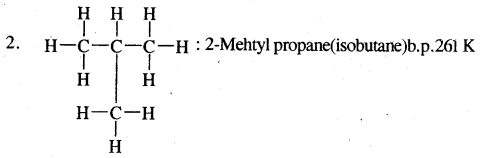
They are called Chain Isomers
Similarly, C5H12 have the following three structures
1. CH3-CH2-CH2-CH2-CH3
: Pentane (n-pentane) b.p. 309 K
2. 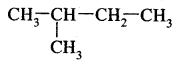
: 2-Methyl butane (isopentane) b.p 301K
3. 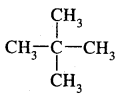
: Dimethylpropane (neopentane) b.p. 282 K.
1, 2, 3 are the chain isomers of pentane. They differ in their boiling points and other properties, though they have the same molecular formula. This difference in properties is due to the difference in their structures, they are termed Structural Isomers.
Preparation of Alkanes:
Petroleum and natural gas are the main sources of alkanes. However, alkanes can be prepared by the following methods.
1. From unsaturated hydrocarbon by hydrogenation.
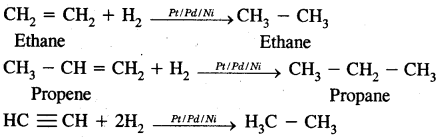
2. From alkyl halides:
1. Alkyl halides (except fluorides) on reduction with zinc and dilute hydrochloric acid give alkanes,
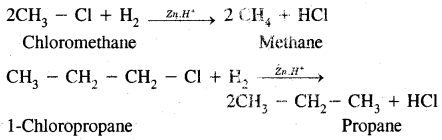
2. By Wurtz reaction: Alkyl halides on treatment with sodium in dry ether give higher alkanes. This method is used to prepare higher alkanes containing an even number of carbon atoms.
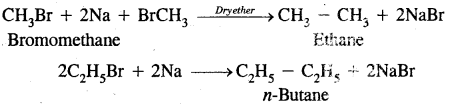
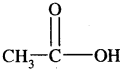
3. From carboxylic acids
1. Sodium salts of fatty acids on heating with soda-lime [a mixture of NaOH + CaO] give alkanes. The process is called decarboxylation [Removal of a molecule of CO2]
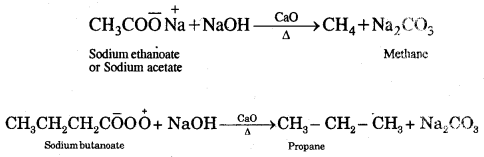
2. Kolbe’s electrolytic method: An aqueous solution of sodium or potassium salt of a carboxylic acid on electrolysis gives alkanes containing an even number of carbon atoms.
![]()
Properties Of Alkanes
(A) Physical Properties:
- Alkanes are almost non-polar due to the covalent nature of C-C and C—H bonds and due to very little difference of electronegativity between C and H atoms. Therefore, they are insoluble in water but soluble in organic solvents.
- Due to weak van der Waals forces, the first four members (from C1 to C4) are gases. The next thirteen (C5 to C17) are liquids and those containing 18 carbon atoms or more solids at 298 K.
- They are colourless and odourless.
- Their boiling points increase with the increase in molecular mass as shown in the table below.
Table: Variation of melting point and boiling point in alkanes
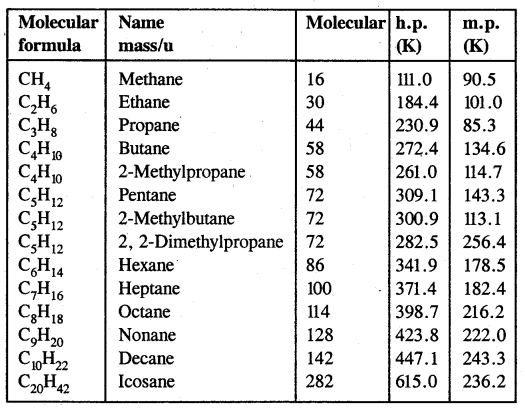
It is due to fact that intermolecular van der Waals forces increase with the increase in molecular size or surface area of the molecules. For example, among the isomeric pentanes.
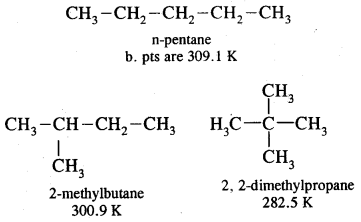
B. Pt of n-pentane is highest (309.1 K), whereas that of 2, 2- dimethyl propane is the lowest (282.5 K). With the increase in the number of branched chains, the molecule attains the shape of a sphere. This results in decreased surface area and hence weaker intermolecular van der Waals forces thus lowering the boiling points.
Chemical Properties Of Alkanes
1. Substitution Reaction: Halogenation
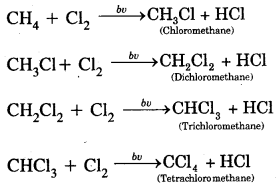
Order of reactivity of halogens is F2 > > Cl2 > Br2 > I2
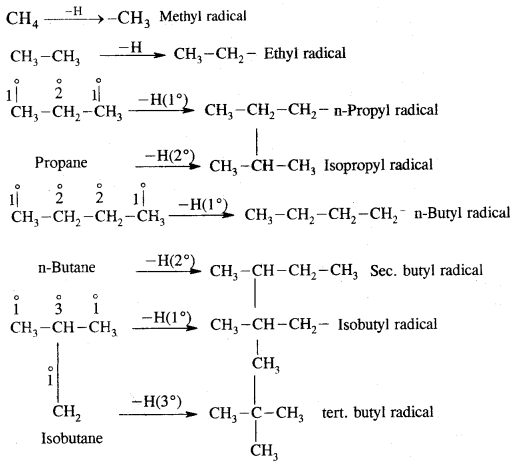
Rate of replacement of hydrogens of alkanes is: 3° > 2° > 1°
Fluorination is too violent to be controlled.
Bromination is similar. Iodination is very slow and a reversible reaction. It can be carried out in the presence of some oxidising agents like HNO3 or HIO3.
CH4 + I2 ⇌ CH3I + HI
HIO3 + 5HI ⇌ 3I2 + 3H2O
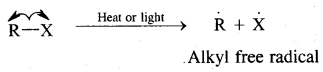
Substitution of halogens in alkanes proceeds via a free-radical mechanism.
2. Combustion: Alkanes on heating in the presence of air or oxygen are completely oxidised to carbon dioxide and water with the evolution of a large amount of heat.
CH4(g) + 2O2 → CO2(g) + 2H2O (l); ΔcH°=- 890 kJ mol-1
C4H10(g) + 6\(\frac{1}{2}\)O2(g) → 4 CO2(g) + 5H2O (1); ΔcH° = -2876 kJ mol-1
Due to the evolution of large amount of heat during combustion, alkanes are used as fuels.
During incomplete combustion in insufficient supply of air or Oxygen, carbon black is formed.
![]()
3. Controlled Oxidation: In a regulated supply of air or oxygen at high pressure and in the presence of suitable catalysts, alkanes give a variety of oxidation products.
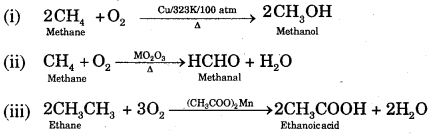
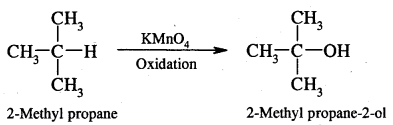
(iv) Ordinarily alkanes resist oxidation but alkanes having tertiary H atoms can be oxidised to corresponding alcohols by KMnO4.
4. Isomerisation: n-Alkanes on heating in the presence of anhydrous aluminium chloride and hydrogen chloride gas isomerises to branched-chain alkanes.
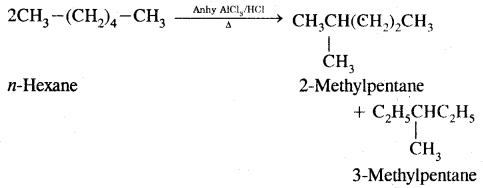
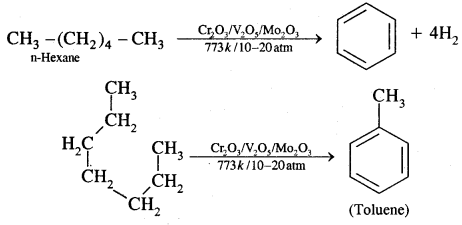
5. Aromatisation: n-alkanes having six or more C atoms on heating to 773 K at 10-20 atmospheric pressure in the presence of oxides of V, Mo or Cr supported over alumina gel dehydrogenated and cyclised to benzene and its homologues. This reaction is termed Aromatisation or reforming.
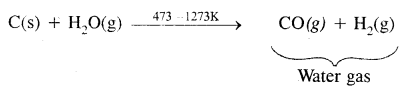
6. Reaction with steam
![]()
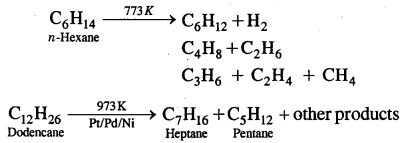
7. Pyrolysis: Higher alkanes on thermal decomposition give lower alkanes, & a mixture of alkanes. The process is also called Cracking.
Conformations:
Alkanes contain C-C sigma (a) bonds. Free-rotation around C – C bond is possible. Such different Spatial, arrangement of atoms obtained by rotation around the C — C bond is called Conformations or Conformers or Rotamers.
Ethane (C2H6) has two major conformational isomers amongst several spatial arrangements differing from each other by a small energy barrier.
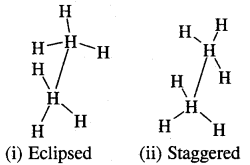
One is called eclipsed form which is less stable as it is associated with more energy [due to repulsion of electrons] and the other is called staggered form which is more stable as it is associated with lower energy. Any other intermediate confrontation is called a skew form.
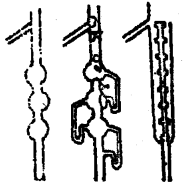
Eclipsed and staggered forms of ethane (C2H5) can be represented by Sawhorse and Newman Projections as shown below of all the conformations of ethane.
1. Sawhorse Projections
Sawhorse projections of change
2. Newman’s Projections
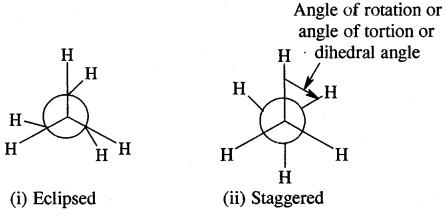
Newman projections of ethane
The staggered form has the least torsional strain and the eclipsed form the maximum torsional strain. The energy difference between the two extreme forms is of the order of 12.5 kJ mo-1 which is very small. These forms have not been separated.
Alkenes. Alkenes are unsaturated hydrocarbons containing at least one
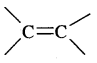
(double bond) or —C = C— (Triple bond)
They are also called Olefins. The general formula of alkenes is CnH2n.
Structure of Double Bond
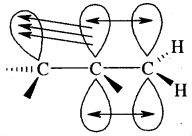
Cabon atoms constituting a double bond undergo sp2 hybridisation. The double bond contains one strong sigma (a) bond and one weak Pi (π) bond. The electrons of the π bond are delocalised and is thus a source of electrons. Any electrophile can come and attack it. That is why alkenes undergo electrophilic addition reactions.
The double bond is shorter in bond length (134 pm) than the C-C single bond (154 pm), π bond is a weaker bond due to poor overlapping between the two 2p orbitals. The strength of the double bond (bond enthalpy 681 kJ mol-1) is greater than that of a C—C single bond (bond enthalpy 348 kJ mol-1) in ethane.
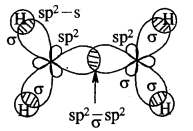
Orbital picture of ethene depicting bonds only
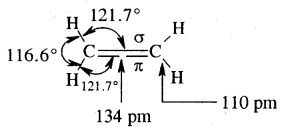
Original picture of ethene showing formation of (a) π-bond. (b) π-cloud and (c) bond angles and bond lengths
Nomenclature of Alkenes:
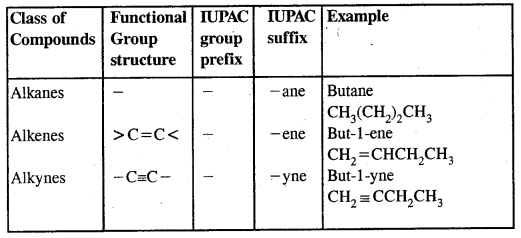
In the IUPAC system, the longest chain of carbon atoms containing the double bond is ‘selected’. The numbering of the chain is done from the end which is nearer to the double bond. The suffix ‘ene’ replace ‘ane’ of alkanes.
Put n = 2 in Cn H2n; C2H4 or H2C = CH2 is ethylene (common name) and ethene in IUPAC system.
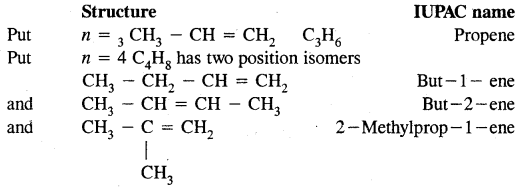
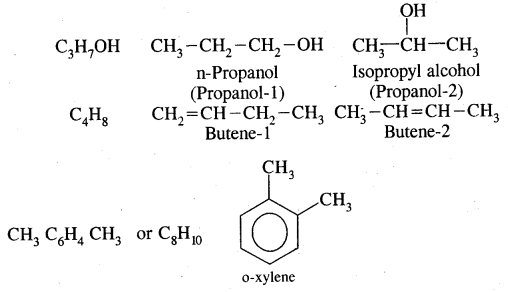
Isomerism in Alkenes
Alkenes show both structural isomerism and geometrical isomerism.
(a) Chain isomerism
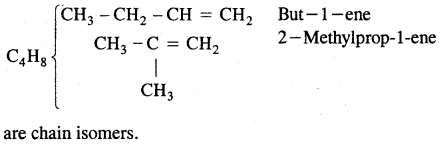
(b) Position isomerism
CH3 – CH2 – CH = CH2 But -1-ene
and CH3 – CH = CH – CH3 But-2-ene
are position isomers as they differ in the position of the functional group.
(c) Geometrical isomerism
Cxy = Cxy and Cxy type of alkenes show geometrical isomerism
e.g. But-2-ene CH3 – CH = CH – CH3 exists in two forms- called geometrical or cis-trans isomers as shown below.

When the identical atoms or groups lie on the same side of the double bond it is called cis-isomer
When the identical atoms or groups lie on the opposite side of the double bond it is called trans-isomer.
The restricted rotation of atoms or groups around the doubly bonded carbon atoms gives rise to different geometries to such compounds. The stereoisomers of this type are called geometrical isomers.
Due to different Spatial arrangements of atoms or groups, geometrical isomers differ in their properties like m.p., b.p., dipole moment, solubility etc.
Cis-form of but-2-ene is more polar than the transform. (Dipole moment) p of cis-form is 0.35 Debye whereas p of transform is almost zero, or trans-2-butene is non-polar. In the transform, two methyl groups being in opposite directions cancel polarities due to each C – CH3 bond.
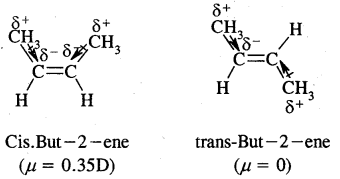
In the case of solids, it is found that the trans isomer has higher m.p. than cis form. This is due to the better symmetry of the trans-isomers. Trans solids fit well into the crystal lattice.
Preparation 0f Alkenes
1. From Alkynes: Alkynes on partial reduction with a calculated amount of dihydrogen in the presence of partially deactivated palletised charcoal called Lindlar’s Catalyst to give cis-alkenes. However, alkynes on reduction with sodium in liquid ammonia form trans-alkenes.
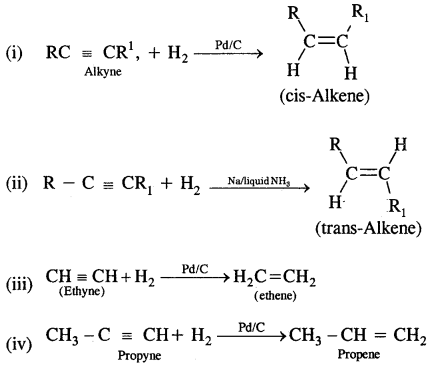
2. From Alkyl Halides: Alkyl halides on heating with alcoholic potash (potassium hydroxide dissolved in alcohol) undergo dehydrohalogenation to give alkenes. This is an example of a β-Elimination reaction.
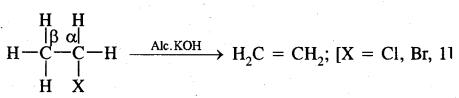
For halogens, the rate of reaction is Iodine > bromine > chlorine while for alkyl groups, it is tert > sec > prim.
3. From vicinal dihalides: Vicinal (on two adjacent C atoms) dihalides on treatment with zinc undergo dehalogenation to give alkenes.
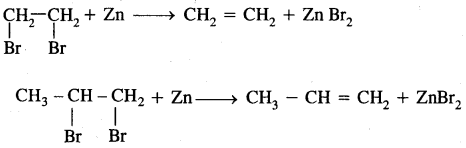
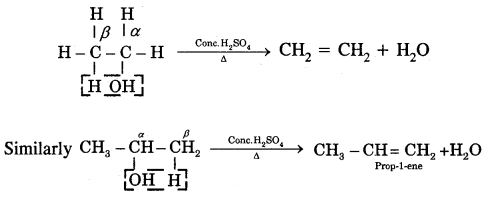
4. From the acidic dehydration of alcohols: Alcohols on heating with conc. H2SO4 lose a molecule of H2O (β-elimination reaction) to form an alkene.
Properties of Alkenes:
Physical properties:
- The first three members are gases, the next 14 are liquids and the higher ones are solids.
- Except for ethene, which has a pleasant smell, all alkenes are odourless and colourless.
- They are insoluble in water but fairly soluble in non-polar solvents like benzene, petroleum, ether etc.
- They show a regular increase in b.p. with an increase in size [For every — CH2— group added b.p. increases by 20—30 K] Like alkanes, straight-chain alkenes have higher b.p. than isomeric branched ‘ alkenes.
- Like alkanes, alkenes are generally non-polar but certain, alkenes are weakly polar due to their unsymmetrical geometry.
Chemical Properties:
(a) Addition reactions:
1. Addition of H2 (catalytical hydrogenation)
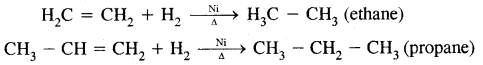
2. Addition of halogens: [Electrophilic addition] Br2 is a reddish-orange liquid that adds to the unsaturated site to give a colourless product. This reaction is used as a test of unsaturation.
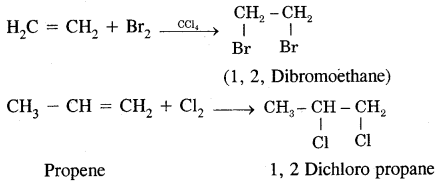
3. Addition of hydrogen halides
The order of reactivity is HI > HBr > HCl
CH2 = CH2 + H – Br → CH3 – CH2Br
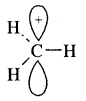
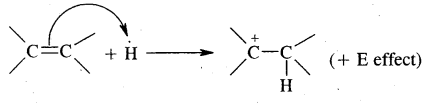
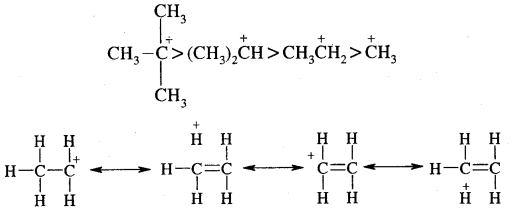
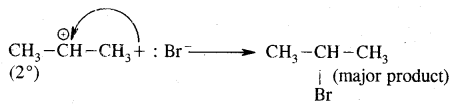
Markovnikov Rule. [Addition of HX to unsymmetric alkenes] “The negative part of addendum (the molecule to be added) goes to that carbon atom of the unsymmetrical alkene which is attached to lesser number of carbon atoms”.
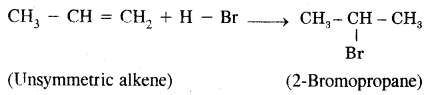
The modern version of Markovnikov Rule. The product is formed from the more stable carbocation.
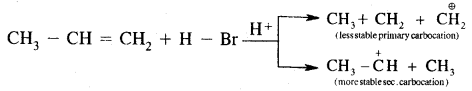
The more stable carbocation [which predominates because it is former faster] reacts with Br- to form the product.
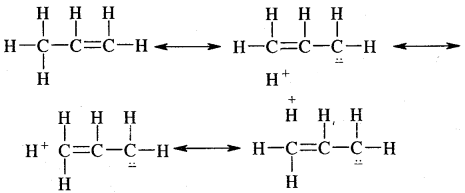
Anti-Markovnikov Addition or Peroxide/Kharash Effect
In the presence of peroxide, the addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov rule. This happens only with HBr but not with HCl and HI. This addition reaction was observed by M.S. Kharash and F.R. Mayo in 1933 at the University of Chicago. This reaction is known as peroxide or Kharash effect or addition effect or addition reaction anti to Markovnikov rule.
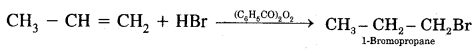
Mechanism: Peroxide effect proceeds via free radical chain mechanism as given below:
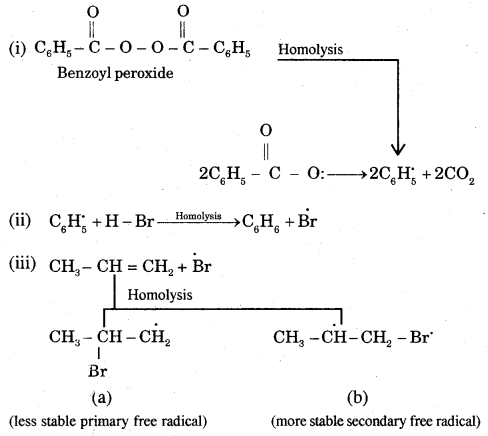
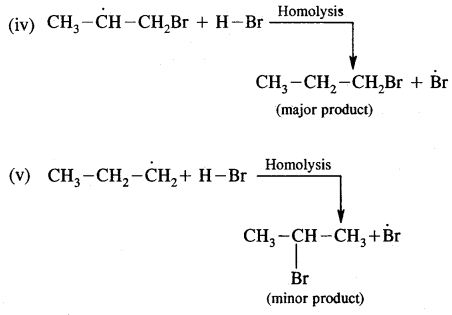
The secondary free radical obtained in the above mechanism (iii) is more stable than the primary. This explains the formation of 1 — bromopropane as the major product. It may be noted that the peroxide effect is not observed in addition to HCl and HI.
This may be due to the fact that the H – Cl bond being stronger (430.5 kJ mol-1) than H — Br bond (363.7 kJ mol-1), is not cleaved by the free radical, whereas the H – I bond is weaker (296.8 kJ mol-1) and iodine free radicals combine to form iodine molecules instead of an addition to the double bond.
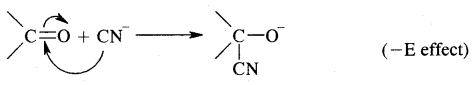
4. Addition of sulphuric acid. Cold, concentrated sulphuric acid adds to alkenes in accordance with the Markovnikov rule as a result of electrophilic addition to form alkyl hydrogen sulphate.
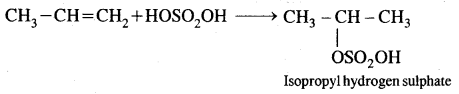
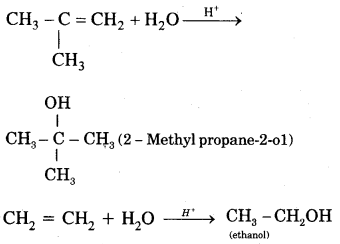
5. Addition of water. In the presence of a few drops of the cone. H2SO4, alkenes undergo hydration with water in accordance with the Markovinkov rule to form alcohols.
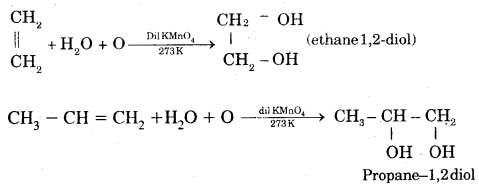
6. Oxidation: (a) Alkenes on reaction with cold, dilute, 1 % alkaline potassium permanganate (KMnO4) solution called Baeyer’s Reagent produce vicinal glycols. The colour of KMnO4 is discharged, It is also used as a test of unsaturation.
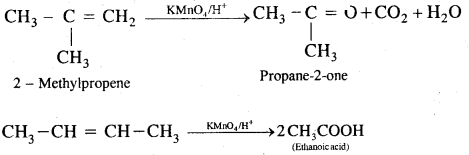
(b) Acidic KMn04 or acidic K2Cr2O7 oxidizes alkenes to ketones and/or acids depending upon the nature of the alkene and the experimental conditions
7. Ozonolysis. It involves the addition of O3 molecules to the alkene to form ozonide followed by cleavage by Zn/H2O to form aldehydes and ketones.
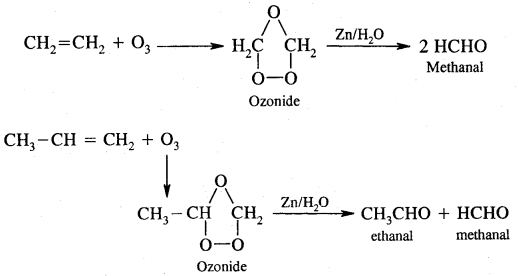
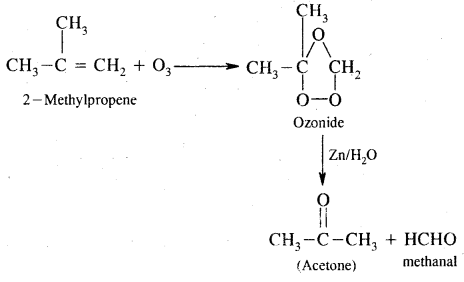
8. Polymerisation. When a large number of ethene molecules combine at high temperature, high pressure in the presence of a catalyst, Polythene is obtained.
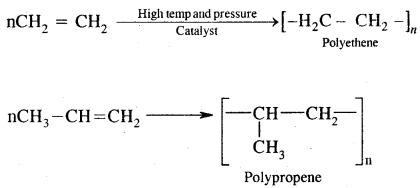
These polymers are of great use in the manufacture of plastic bags, squeeze bottles, toys, pipes radio and TV cabinets, milk crates, plastic buckets and other moulded articles.
Alkynes: Alkynes are unsaturated hydrocarbons containing at least one — C = C — triple bond.
General formula: CnH2n-2
Common & I.U.P.A.C. names of Alkynes
n = 2 C2H2 H – C ≡ C – H Acetylene Ethyne
n = 3 C3H4 CH3 — C ≡ CH MethylacetylenePropyne
n = 4 C4H6
- CH3CH2C = CH Ethylacetylene But-l-yne
- CH3 – C = C-CH3 Dimethylacetylene But-2-yne
n = 5 C5H8
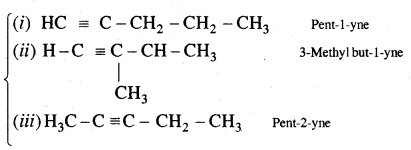
Structures (i) and (iii) are position isomers
Structures (i) and (ii) and (iii) are chain isomers
Structure of Triple Bond.
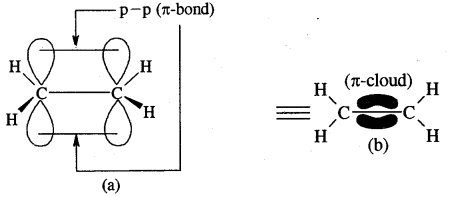
Each carbon atom of ethyne has two sp hybridized orbitals. Carbons-carbon sigma (a) bond is obtained by the head-on overlapping of the two sp hybridised orbitals of the two carbon atoms. The remaining sp hybridised orbitals of the two carbon atoms. The remaining sp hybridized orbital of each carbon atom undergoes overlapping along the internuclear axis with the Is orbital of each of the two hydrogen atoms forming two C — H sigma bonds. H – C—C bond angle is 180°.
Each carbon has two unhybridised p orbitals which are perpendicular to each other as well as to the plane of the C – C sigma bond. The 2p orbitals of one carbon atom are parallel to the 2p orbitals of the other carbon atom, which undergo lateral or sideways overlapping to form two pi (p) bonds between two carbon atoms. Thus ethyne molecule consists of one C — C s bond, two C >- Hs bonds and two C — C p bonds.
The strength of the C = C bond (bond enthalpy 823 kJ mol-1) is more than those of the C = C bond (bond enthalpy 681 kJ mol-1) and C – C bond (bond enthalpy 48 kJ mol-1). The C = C bond length is shorter (120 pm) than those of C = C (134 pm) and C – C) (154 pm). The electron cloud between two carbon atoms is cylindrically symmetrical about the internuclear axis. Thus ethyne is a linear molecule.
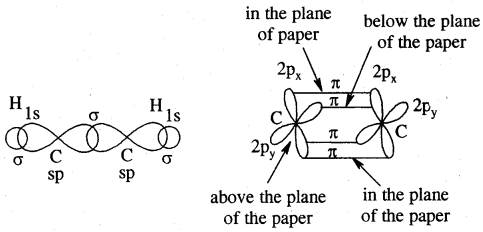
Orbital picture of ethyne showing (a) sigma overlaps (b) pi overlaps bond angles and bond lengths.
Preparation of Acetylene (Ethyne). Commercially, it is prepared by the action of water on calcium carbide.
CaC2 + 2H2O → Ca(OH)2 + C2H2 (Ethyne)
2. From Vicinal Dihalidies. Vicinal dihalides on treatment with alcoholic potassium hydroxide undergo dehydrohalogenation.
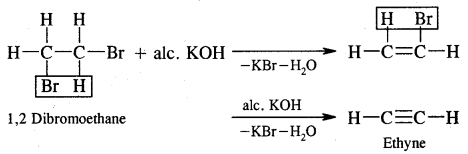
Properties of Alkynes:
Physical properties.
- First, three members are gases, the next eight are liquids and the higher ones are solids.
- All alkynes are colourless.
- Except for enthene which has a characteristic odour, others are odourless.
- Alkynes are weakly polar in nature.
- They are lighter than water and immiscible with water but soluble in organic solvents like ethers, benzene etc.
- Their m, p., b, p, and density increases with an increase in molar mass.
Chemical Properties: Alkynes show usual addition reactions, acidic reactions and polymerisation reaction.
A. Acidic character of alkynes: Unlike alkenes, ethyne shows acidic reactions.
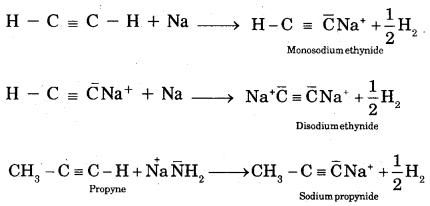
Alkanes, alkenes, and alkynes follow the following trend in their acidic behaviour.
- H – C ≡ C – H > CH2 = CH2 > CH3 – CH3
- H – C ≡ C – H > CH3 – C ≡ CH > > CH3 – C ≡ C – CH3
B. Addition reactions,
1. Addition of dihydrogen.
Alkynes contain a triple bond. Therefore, they add up two molecules of H2.
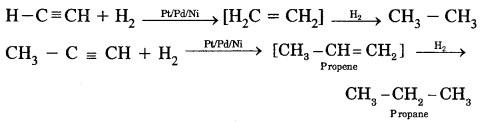
2. Addition of Halogens. When Br2 is added to alkynes, the reddish-orange colour of Br2 disappear. It is a test of unsaturation.
![]()
3. Addition of hydrogen halides [HCl, HBr, HI]
Two molecules get added to alkynes to form gem dihalides.
![]()
4. Addition of water
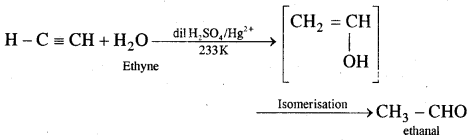
5. Polymerisation
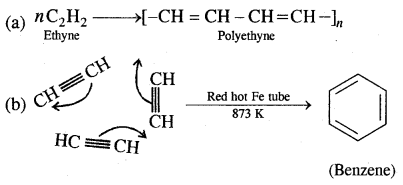
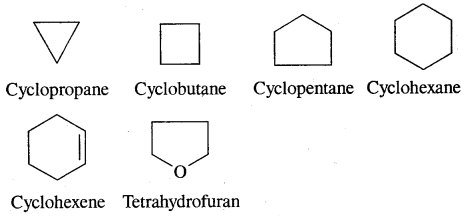
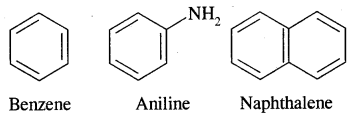
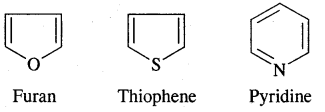
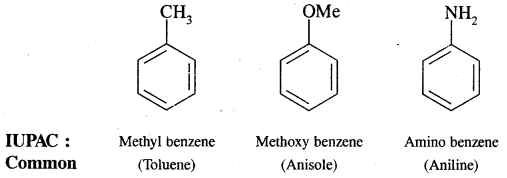
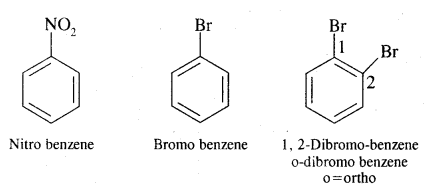
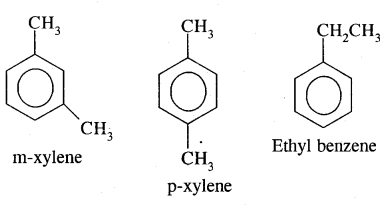
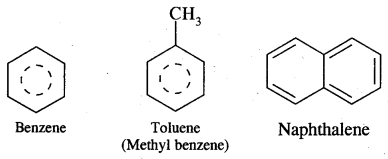
Aromatic Hydrocarbons or Arenes: Aromatic compounds containing benzene ring are known as Benzenoids and those not containing a benzene ring are called Non-Benzenoids. Some of the arenas are given below.
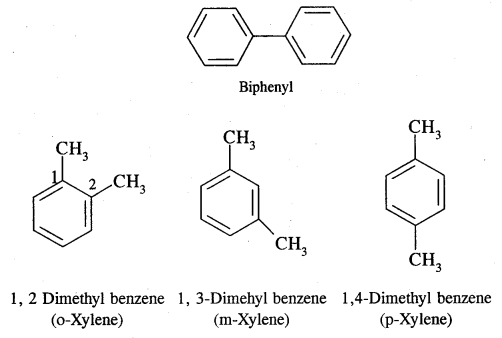
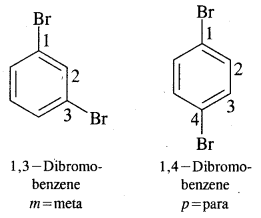
Where o = ortho (1,2)
m = meta (1, 3)
p = para (1, 4)
Structure of Benzene:
- Molecular formula C6H6 indicates that benzene is an unsaturated hydrocarbon.
- The unusual stability of benzene and no change of orange-red colour of Br2 in addition to benzene ruled out the open chain structure of benzene.
- It forms a triozonide which indicates the presence of three double bonds.
- Benzene produces one and only one monosubstituted derivative which indicates that all the six-carbon and six hydrogen atoms of benzene are identical.
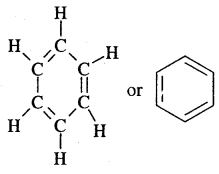
- A. Kekule’ in 1865 proposed the cyclic structure for benzene with alternate single and double bonds in carbon atoms with each C atom carrying one hydrogen.
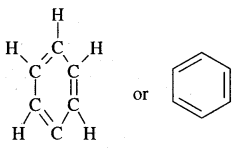
Kekule’ suggested the oscillating nature of double bonds.

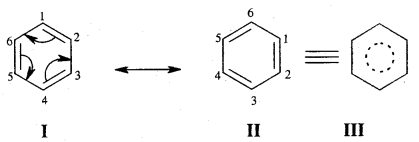
Resonance and Stability of Benzene
Benzene is a resonance hybrid of various, resonating structures. The two structures are given above by Kekule’ are the main contributing st; lectures. The hybrid structure is (c) is given below.
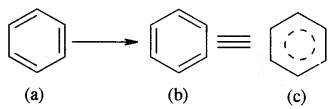
The circle represents the six electrons that are delocalised between the six carbon atoms of the benzene ring.
Orbital Picture of Benzene.
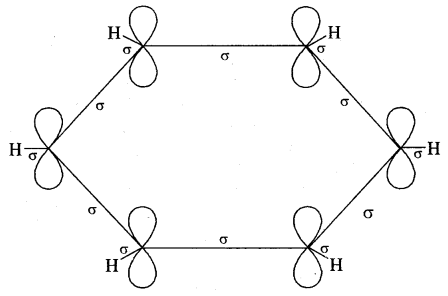
All the six carbon atoms of benzene are sp2 hybridised. Two of these three sp2 hybrid orbitals of each C atom overlap with sp2 hybrid orbitals of adjacent C atoms to form six C – C single bonds which are in the hexagonal plane. The remaining sp2 orbital of each C atom overlaps with the s-orbital of each hydrogen atom to form six C — H single sigma bonds. Each C atom is now left with one unhybridised p- orbital perpendicular to the plane of the ring as shown on the next page.
The unhybridised p orbital of carbon atoms is close enough to form π (Pi) bond by sidewise overlap. These overlaps can be of overlaps of p-orbitals of C1 — C2, C3 — C4, C5 – C6 or C3, C4 — C5, C6 – C1 respectively as shown in the following figures.
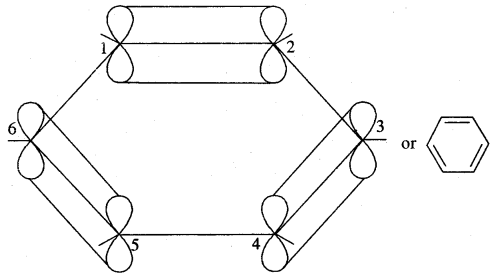
(a)
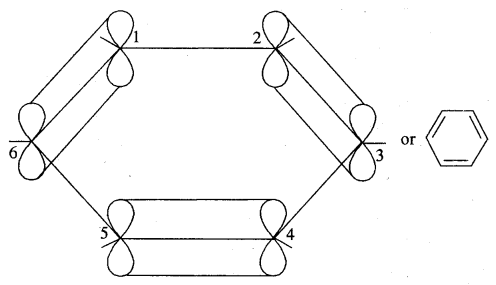
(b)
X-ray diffraction data reveals that benzene is a planar molecule. The six n electrons are delocalised and spread on the whole of the molecule: one half of the electron cloud above and the other half below the plane of the benzene ring. The presence of delocalised n electrons in benzene makes it more stable than the imaginary cyclohexatriene.
or
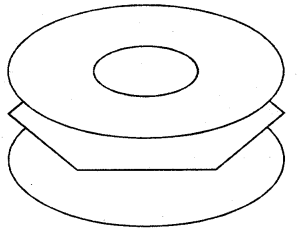
(Electron cloud)
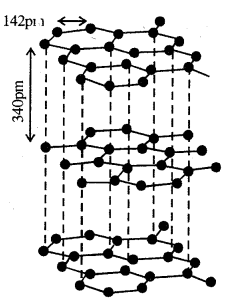
If there were three single C – C bonds and three alternate C = C bonds present in benzene, the bond lengths should have been 154 pm and 134 pm respectively. In benzene there are neither C – C double bonds present as all the six C — C bonds in benzene are exactly alike and have a bond length of 139 pm. Thus the absence of pure double bonds in benzene accounts for the hesitation on the part of benzene to take part in additional reactions. Due to its extra stability, it prefers to show substitution reactions.
Aromaticity: Benzene is considered a parent aromatic compound. Now the name is applied to all the ring systems whether or not having benzene ring, possessing the following characteristics.
- It should be planar
- Complete delocalisation of % electrons in the ring.
- Presence of (4n + 2) n electrons in the ring where n is an integer (n = 0, 1, 2). This is called Huckel Rule.
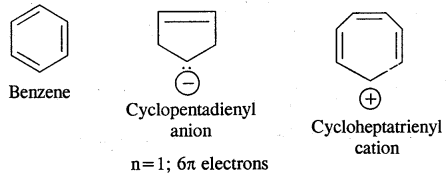
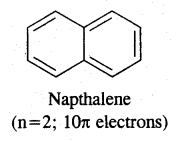
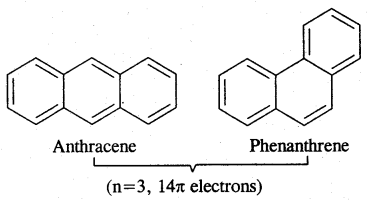
Preparation of Benzene
Benzene is commercially isolated from the ‘Light oil fraction’ of coal tar. However, it may be prepared in the laboratory by the following methods.
1. From ethyne
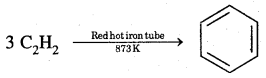
2. Decarboxylation of the aromatic acids Sodium salt of benzoic acid on heating with soda lime gives benzene.
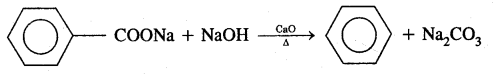
3. Reduction of Phenol in the presence of zinc dust gives benzene
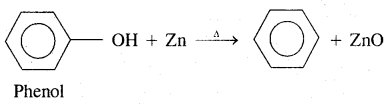
Properties of Benzene (Aromatic hydrocarbons)
Physical properties
- Aromatic hydrocarbons are non-polar.
- They are colourless liquids or solids with a characteristic aroma.
- Aromatic hydrocarbons are immiscible with water but are readily miscible with organic solvents.
- They burn with a sooty flame.
Chemical Properties
Arenes undergo electrophilic substitution reactions. However, under special conditions, they undergo addition and oxidation reactions.
Electrophilic Substitution Reactions of arenes are nitration, halogenations, sulphonation, Friedel Craft’s reactions.
In all these reactions, the attacking reagent is an electrophile E®.
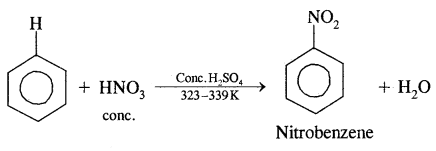
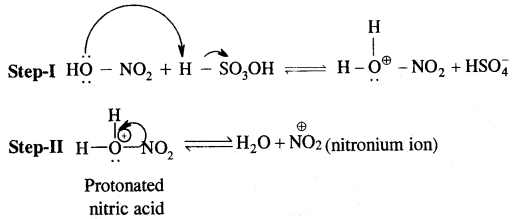
1. Nitration.
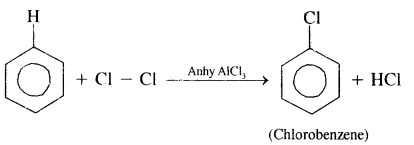
2. Halogenation: Arenes react with halogen in the presence of Lewis acids like FeCl3, FeBr3, or AlCl3 to yield halo arenes.
Order of reactivity of halogens is Cl2 > Br2 > I2.
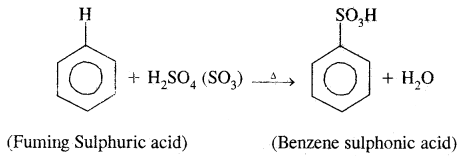
3. Sulphonation: Here H of the benzene ring is replaced by sulphonic group (— SO2 OH). It is carried out by heating benzene with fuming sulphuric acid (oleum).
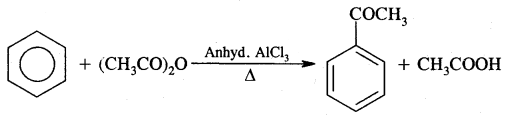
4. Friedel Craft’s Reaction:
(a) Alkylation: On reacting benzene with an alkyl halide in the presence of anhydrous Aluminium chloride, alkyl benzene is formed.
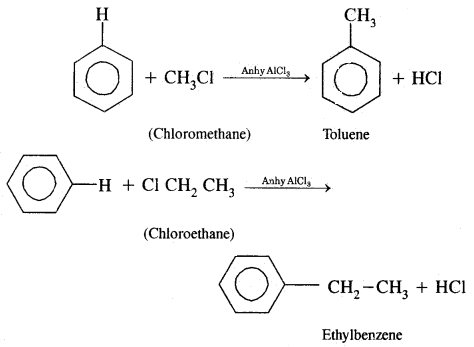
(B) Acylation: On treating benzene with an acyl chloride in the presence of Lewis acids (AlCl3) gives acyl benzene.
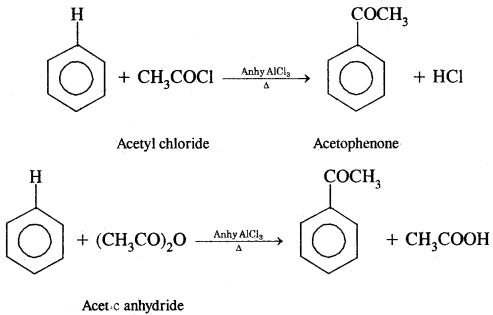
Mechanism of electrophilic substitution reactions: It involves three steps:
- Generation of an electrophile.
- Formation of a resonance-stabilised carbocation intermediate.
- Removal of proton H+ to form the product.
1. Generation of Eelecrophile (E+): In the above reactions electrophiles like Cl+ (chloronium ion) is generated during chlorination by reacting with any. AlCl3.
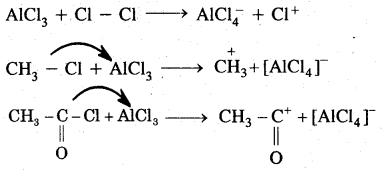
In the case of Nitration, NO2 (nitronium ion) is generated.
2. Formation of carbocation (arenium ion) results with one of the carbon getting sp3 hybridised on the attack of the electrophile (E)+
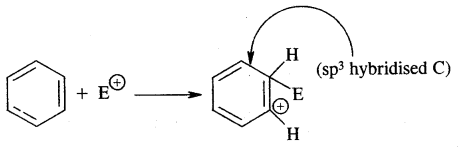
Sigma complex (arenium ion)
The intermediate arenium ion gets stabilised by resonance.
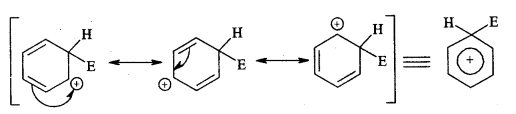
3. Removal of a proton (H+)
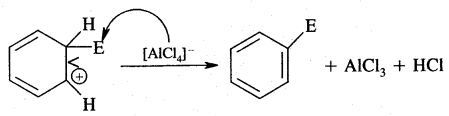
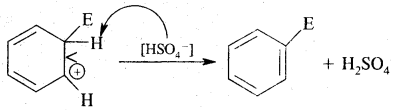
2. Addition reactions: Under drastic conditions of high temperature and or pressure in the presence in the presence of nickel catalyst, dihydrogen gets added to the benzene.
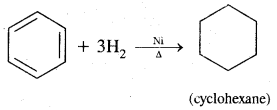
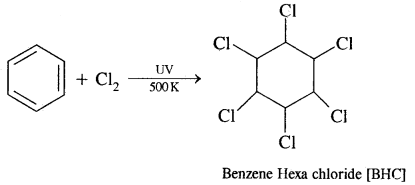
In the presence of ultraviolet light, three molecules of Cl2 get added to benzene to form Benzene hexachloride [BHC] C6H6C16 also called Gammaxene.
3. Oxidation by combustion: When heated in air, benzene burns with a sooty flame producing CO2 and H2O.
C6H6 + O2 → 6 CO2 + 3H2O
General combustion reaction for any hydrocarbon is
CxHy +(x + y/4)O2 → xCO2 + y/2H2O.
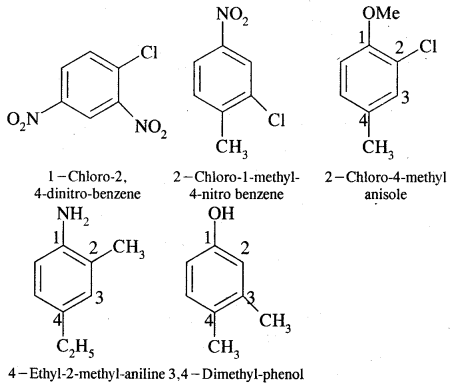
Directive Influence of a Functional Group in Monosubstituted Benzene
Ortho and para directing groups: The groups which direct the incoming group to ortho & para positions are called ortho & para directing groups. In phenol, for example, — OH (hydroxy) group attacked to benzene directs the new (or coming group) to ortho para positions as explained below:
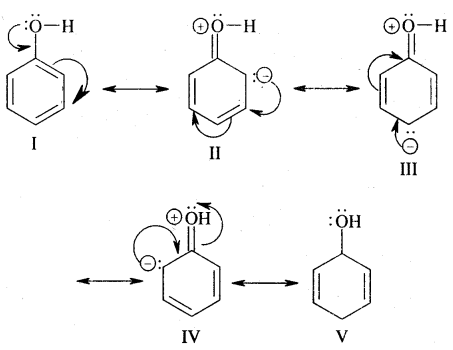
From the above structures, it is clear that electron density is more at ortho & para positions (structure II, III & IV) to the – OH group. Hence the coming electrophile will prefer to attack ortho & para position rather than meta. However due to the — I effect exerted by the — OH group, electron density at o—&p—position is slightly reduced. But overall, there is an increase of electron density ato-Scp- position. Hence the substituent at o—&p — positions to the -OH group.
Therefore, the -OH group is an activating group, as it activates the benzene ring for the attack of an electrophile. Other activating groups are NH2, -NHR, NHCOCH3, -OCH3, -CH3, -C2H5 etc.
Halogens are a class among themselves. They are deactivating and at the same time o—Scp — directing. Because of the, I effect, the overall electron density on benzene decreases. It makes further substitution difficult. However, due to resonance, the electron density on the o—& p — position is greater than at the meta position. Hence they are also o— & p — directing.
Meta-directing groups. The groups which when present in the benzene ring direct the incoming groups to meta position are called meta-directing groups. Some of the meta-directing groups are
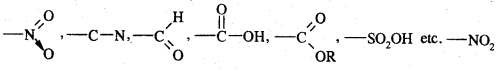
(nitro group), for example, reduces the electron density in the benzene ring due to its — I effect. Nitrobenzene is a resonance hybrid of the following five canonical structures.
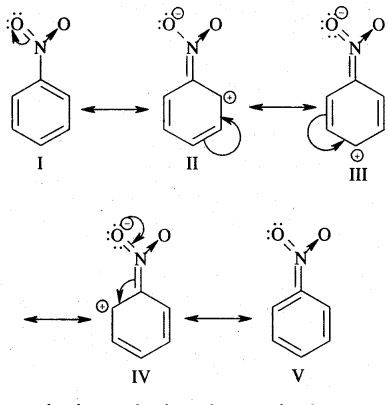
In this case, the electron density on the benzene ring decreases making further substitution difficult. Therefore these groups are called deactivating groups. The electron density on the o – and p – position is comparatively less than that at the meta position. Hence, the electrophile attacks on comparatively electron-rich meta position, resulting in meta-substitution.
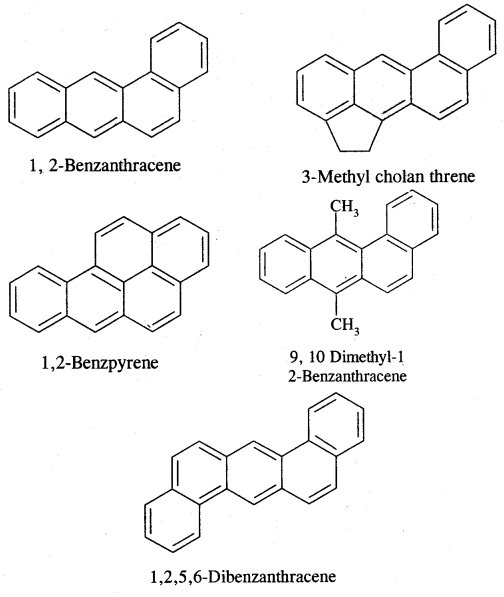
Carcinogenicity and Toxicity:
Benzene and polynuclear hydrocarbons containing more than two fused benzene rings are toxic and said to possess cancer-producing (carcinogenic) property. They enter into the human body and undergo various biochemical reactions and finally damage DNA and cause cancer. Some of the carcinogenic hydrocarbons are given below. Such polynuclear hydrocarbons are formed on incomplete combustion of organic materials like tobacco coal and petroleum.