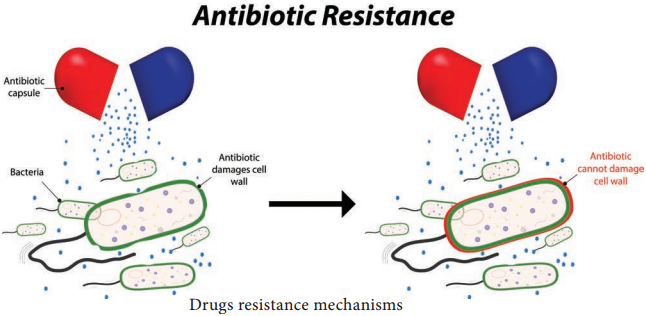
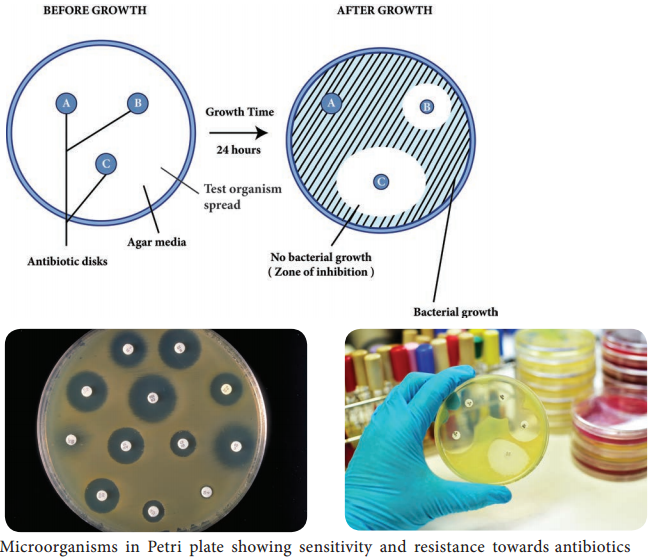
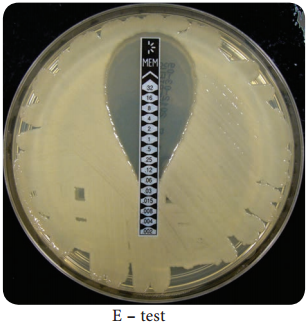
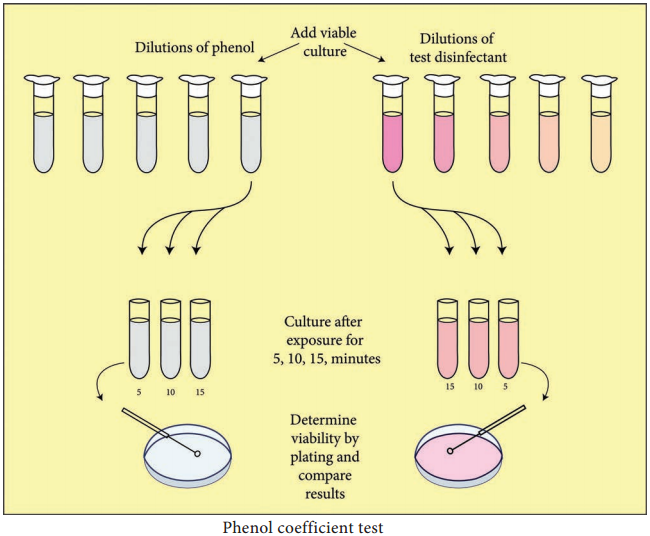
Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Lipid Catabolism
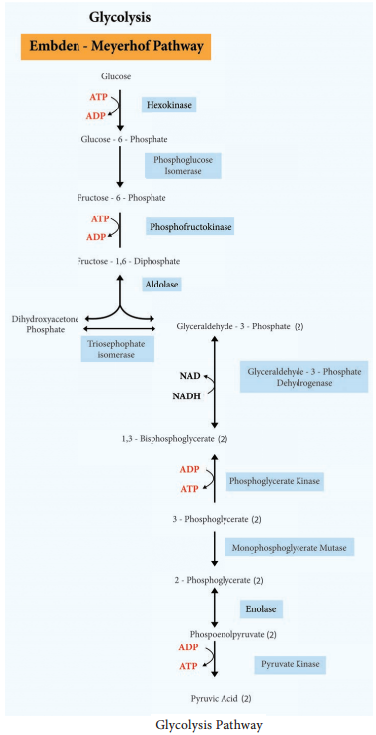
Microorganisms frequently use lipids such as triglyceride or triacylglycerol (esters of glycerol and fatty acids) as common reserve energy sources. These can be hydrolyzed to glycerol and fatty acid by microbial lipases. The glycerol is then phosphorylated and oxidized to Dihydroxyacetone phosphate and then catabolized in the Glycolysis pathway.
Fatty acids from triacylglycerols and other lipids are often oxidized in the β-oxidation pathway. In this pathway fatty acids are degraded to acetyl CoA (2C segment), then it enters into the TCA cycle.
Lipid catabolism comprises two major spatially and temporarily separated steps, namely lipolysis, which releases fatty acids and head groups and is catalyzed by lipases at membranes or lipid droplets, and degradation of fatty acids to acetyl-CoA, which occurs in peroxisomes through the β-oxidation pathway in green.
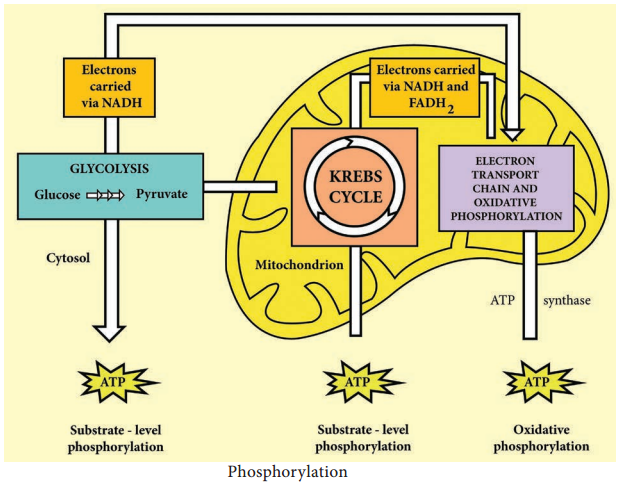
The released fatty acids are catabolized in a process called β-oxidation, which sequentially removes two-carbon acetyl groups from the ends of fatty acid chains, reducing NAD+ and FAD to produce NADH and FADH2, respectively, whose electrons can be used to make ATP by oxidative phosphorylation.
Lipid metabolism begins in the intestine where ingested triglycerides are broken down into smaller chain fatty acids and subsequently into monoglyceride molecules by pancreatic lipases, enzymes that break down fats after they are emulsified by bile salts.
Lipid metabolism is the process that most of the fat ingested by the body is emulsified into small particles by bile and then the lipase secreted by the pancreas and small intestine hydrolyzes the fatty acids in the fat into free fatty acids and monoglycerides.