On this page, you will find Heron’s Formula Class 9 Notes Maths Chapter 7 Pdf free download. CBSE NCERT Class 9 Maths Notes Chapter 7 Heron’s Formula will seemingly help them to revise the important concepts in less time.
CBSE Class 9 Maths Chapter 7 Notes Heron’s Formula
Heron’s Formula Class 9 Notes Understanding the Lesson
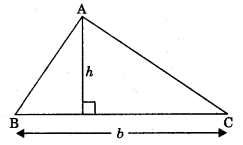
1. Area of triangle with base ‘b’ and altitude ‘h’ is
Area = \(\frac{1}{2}\)(b x h)
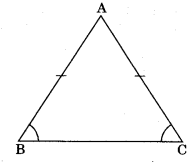
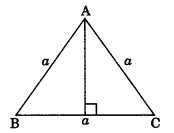
2. Area of an isosceles triangle with equal sides ‘a’ each and third side b is
Area \(=\frac{b}{4} \sqrt{4 a^{2}-b^{2}}\)
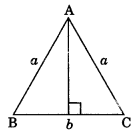
3. Area of an equilateral triangle with side ‘a’ each is
Area=\(\frac{\sqrt{3}}{4} a^{2}\)
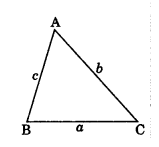
4. Area of a triangle by Heron’s formula when sides a, b and c are given is
Area = \(\sqrt{s(s-a)(s-b)(s-c)}\)
Where s = semi-perimeter = \frac{a+b+c}{2}
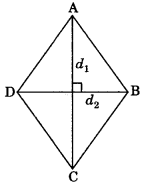
5. Area of rhombus
Area= \(\frac{1}{2} d_{1} \times d_{2}\)
where d1 and d2 are the lengths of its diagonals.
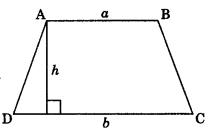
6. Area of trapezium
Area=\(\frac{1}{2}\) (a+b) h
where a and b are parallel sides and h is distance between two parallel sides.