Check the below NCERT MCQ Questions for Class 12 Chemistry Chapter 1 The Solid State with Answers Pdf free download. MCQ Questions for Class 12 Chemistry with Answers were prepared based on the latest exam pattern. We have provided The Solid State Class 12 Chemistry MCQs Questions with Answers to help students understand the concept very well.
Class 12 Chemistry Chapter 1 MCQ With Answers
Chemistry Class 12 Chapter 1 MCQs On The Solid State
Solid State MCQ Question 1.
Which one of the following is non-crystalline or amorphous?
(a) Diamond
(b) Graphite
(c) Glass
(d) Common Salt
Answer
Answer: (c) Glass
Solid State Class 12 MCQ Question 2.
NaCl typecrystal (with coordination no. 6 : 6) can be converted into CsCl type crystal (with coordination no. 8 : 8) by applying
(a) high temperature
(b) high pressure
(c) high temperature and high pressure
(d) low temperature and low pressure
Answer
Answer: (b) high pressure
Solid State MCQs Class 12 Question 3.
How many chloride ions are surrounding sodium ion in sodium chloride crystal ?
(a) 4
(b) 8
(c) 6
(d) 12
Answer
Answer: (c) 6
Class 12 Chemistry Chapter 1 MCQ Question 4.
In NaCl structure
(a) all octahedral and tetrahedral sites are occupied
(b) only octahedral sites are occupied
(c) only tetrahedral sites are occupied
(d) neither octahedral nor tetrahedral sites are occupied
Answer
Answer: (b) only octahedral sites are occupied
Class 12 Chemistry Chapter 1 MCQs Question 5.
In Zinc blende structure
(a) zinc ions occupy half of the tetrahedral sites
(b) each Zn2- ion is surrounded by six sulphide ions
(c) each S2- ion is surrounded by six Zn2+ ions
(d) it has fee structure
Answer
Answer: (c) each S2- ion is surrounded by six Zn2+ ions
Solid State MCQ Questions For Class 12 Question 6.
A unit cell of BaCl2 (fluorite structure) is made up of
(a) four Ba2+ ions and four Cl– ions
(b) four Ba2- ions and eight Cl– ions
(c) eight Ba² ions and four Cl– ions
(d) four Ba² ions and six Cl– ions
Answer
Answer: (b) four Ba2- ions and eight Cl– ions
Class 12 Chemistry MCQ Chapter 1 Question 7.
Alkali halids do not show Frenkel defect because
(a) cations and anions have almost equal size
(b) there is a large difference in size of cations and anions
(c) cations and anions have low coordination number
(d) anions cannot be accommodated in voids
Answer
Answer: (a) cations and anions have almost equal size
MCQ On Solid State Class 12 Question 8.
The coordination number of Y will be in the XY types of crystal:
(a) 6
(b) 8
(c) 12
(d) 4
Answer
Answer: (a) 6
Solid State Chemistry Class 12 MCQ Question 9.
The fraction of the total volume occupied by the atoms present in a simple cube is
(a) \(\frac{π}{4}\)
(b) \(\frac{π}{6}\)
(c) \(\frac{π}{3√2}\)
(d) \(\frac{π}{4√2}\)
Answer
Answer: (b) \(\frac{π}{6}\)
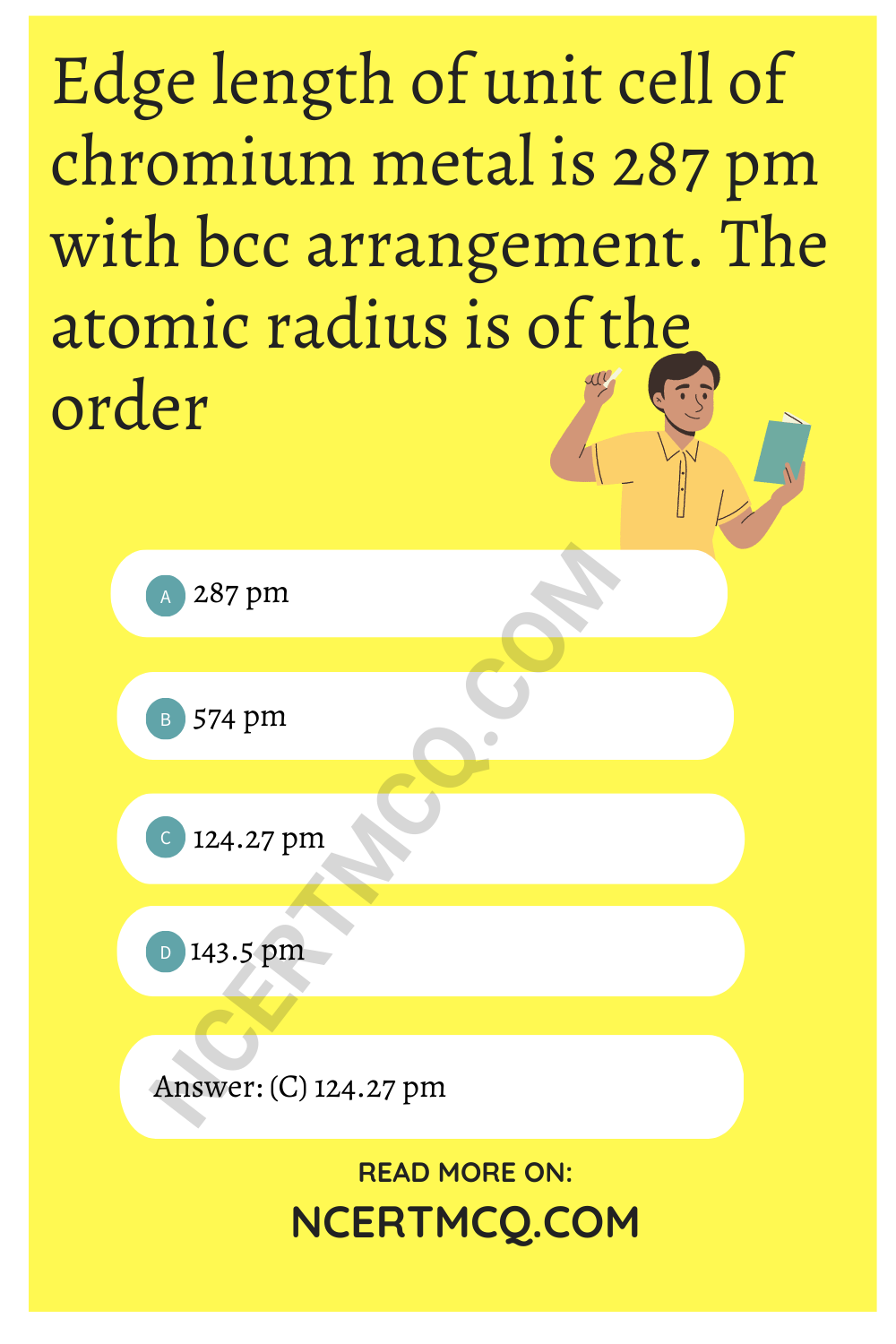
Chemistry Class 12 Chapter 1 MCQ Question 10.
Edge length of unit cell of chromium metal is 287 pm with bcc arrangement. The atomic radius is of the order
(a) 287 pm
(b) 574 pm
(c) 124.27 pm
(d) 143.5 pm
Answer
Answer: (c) 124.27 pm
MCQ Solid State Class 12 Question 11.
The edge length of fee cell is 508 pm. If radius of cation is 110 pm, the radius of anion is
(a) 110 pm
(b) 220 pm
(c) 285 pm
(d) 144 pm
Answer
Answer: (d) 144 pm
Chapter 1 Chemistry Class 12 MCQ Question 12.
The density of a metal which crystallises in bcc lattice with unit cell edge length 300 pm and molar mass 50 g mol-1 will be
(a) 10 g cm-3
(b) 14.2 g cm-3
(c) 6.15 g cm-3
(d) 9.3 2 g cm-3
Answer
Answer: (c) 6.15 g cm-3
Class 12 Solid State MCQ Question 13.
How many lithium atoms are present in a unit cell with edge length 3.5 Å and density 0.53 g cm-3? (Atomic mass of Li = 6.94):
(a) 2
(b) 1
(c) 4
(d) 6
Answer
Answer: (a) 2
MCQ Questions On Solid State With Answers Pdf Question 14.
The distance between Na– and CL– ions in NaCl with a density 2.165 g cm-3 is
(a) 564 pm
(b) 282 pm
(c) 234 pm
(d) 538 pm
Answer
Answer: (b) 282 pm
MCQ Of Solid State Class 12 Question 15.
An element with atomic mass 100 has a bcc structure and edge length 400 pm. The density of element is
(a) 10.37 g cm-3
(b) 5.19 g cm-3
(c) 7.29 g cm-3
(d) 2.14 g cm-3
Answer
Answer: (b) 5.19 g cm-3
Question 16.
Fe3O4 (magnetite) is an example of
(a) normal spinel structure
(b) inverse spinel structure
(c) fluoride structure
(d) anti fluorite structure
Answer
Answer: (b) inverse spinel structure
Question 17.
Which of the following crystals does not exhibit Frenkel defect?
(a) AgBr
(b) AgCl
(c) KBr
(d) ZnS
Answer
Answer: (c) KBr
Question 18.
What type of stoichiometric defect is shown by ZnS?
(a) Schottky defect
(b) Frenkel defect
(c) Both Frenkel and Schottky defects
(d) Non-stoichiometric defect
Answer
Answer: (b) Frenkel defect
Question 19.
Silver halides generally show
(a) Schottky defect
(b) Frenkel defect
(c) Both Frenkel and Schottky defects
(d) cation excess defect
Answer
Answer: (c) Both Frenkel and Schottky defects
Question 20.
Which of the following will have metal deficiency defect?
(a) NaCl
(b) FeO
(c) KCl
(d) ZnO
Answer
Answer: (b) FeO
Question 21.
In which pair most efficient packing is present?
(a) hep and bcc
(b) hep and ccp
(c) bcc and ccp
(d) bcc and simple cubic cell
Answer
Answer: (b) hep and ccp
Question 22.
What is the effect of Frenkel defect on the density of ionic solids?
(a) The density of the crystal increases
(b) The density of the crystal decreases
(c) The density of the crystal remains unchanged
(d) There is no relationship between density of a crystal and defect present in it
Answer
Answer: (c) The density of the crystal remains unchanged
Question 23.
In a Schottky defect
(a) an ion moves to interstitial position between the lattice points
(b) electrons are trapped in a lattice site
(c) some lattice sites are vacant
(d) some extra cations are present in interstitial space
Answer
Answer: (c) some lattice sites are vacant
Question 24.
To get n-type of semiconductor, germanium should be doped with
(a) gallium
(b) arsenic
(c) aluminium
(d) boron
Answer
Answer: (b) arsenic
![]()
Question 25.
p-type semiconductors are formed When Si or Ge are doped with
(a) group 14 elements
(b) group 15 elements
(c) group 13 elements
(d)group 18 elements
Answer
Answer: (c) group 13 elements
Question 26.
Which of the following conditions favours the existence of a substance in the solid state?
(a) High temperature
(b) Low temperature
(c) High thermal energy
(d) Weak cohesive forces
Answer
Answer: (b) Low temperature
We hope the given NCERT MCQ Questions for Class 12 Chemistry Chapter 1 The Solid State with Answers Pdf free download will help you. If you have any queries regarding The Solid State CBSE Class 12 Chemistry MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 12 Chemistry MCQ:
- The Solid State Class 12 MCQ
- Solutions Class 12 MCQ
- Electrochemistry Class 12 MCQ
- Chemical Kinetics Class 12 MCQ
- Surface Chemistry Class 12 MCQ
- General Principles and Processes of Isolation of Elements Class 12 MCQ
- The p-Block Elements Class 12 MCQ
- The d-and f-Block Elements Class 12 MCQ
- Coordination Compounds Class 12 MCQ
- Haloalkanes and Haloarenes Class 12 MCQ
- Alcohols, Phenols and Ethers Class 12 MCQ
- Aldehydes, Ketones and Carboxylic Acids Class 12 MCQ
- Amines Class 12 MCQ
- Biomolecules Class 12 MCQ
- Polymers Class 12 MCQ
- Chemistry in Everyday Life Class 12 MCQ