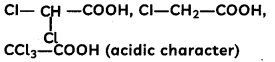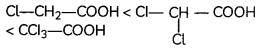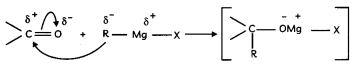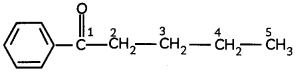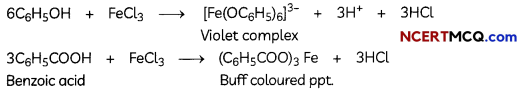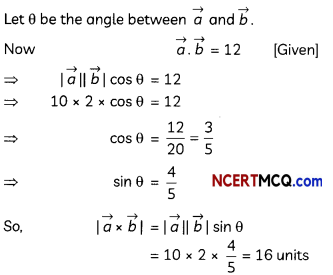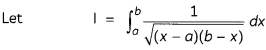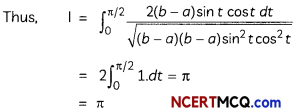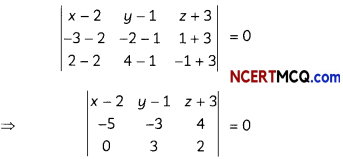Students can access the CBSE Sample Papers for Class 12 Biology with Solutions and marking scheme Term 2 Set 12 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 12 Biology Standard Term 2 Set 12 for Practice
Time Allowed: 2 Hours
Maximum Marks: 40
General Instructions:
- All questions are compulsory.
- The question paper has three sections and 13 questions. All questions are compulsory.
- Section-A has 6 questions of 2 marks each; Section-B has 6 questions of 3 marks each; and Section-C has a case-based question of 5 marks.
- There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
- Wherever necessary, neat and properly labeled diagrams should be drawn.
SECTION – A
(Section A has 6 Questions of 2 marks each.)
Question 1.
(A) Name the property of normal cells, the absence of which in cancerous cells leads to the formation of tumours.
(B) The tumors are classified into two types, in which one of the types remains confined to the original location and the other type of tumours invade neighboring tissues. Name the two types of tumours. (2)
Question 2.
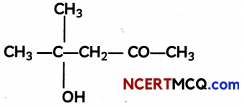
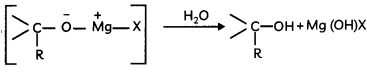
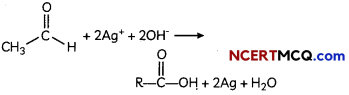
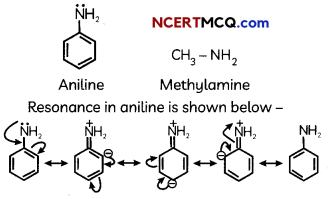
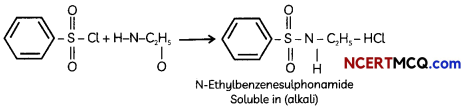
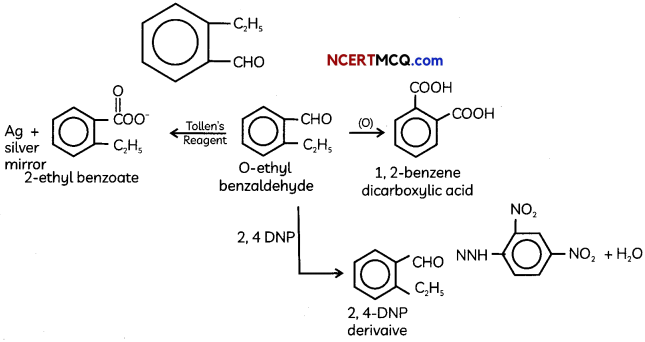
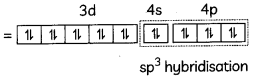
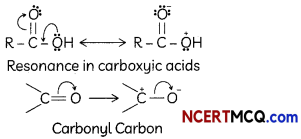
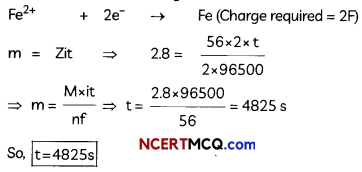
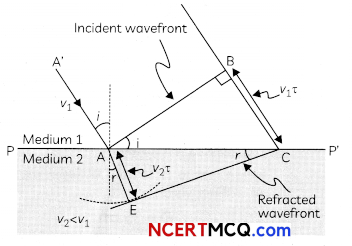
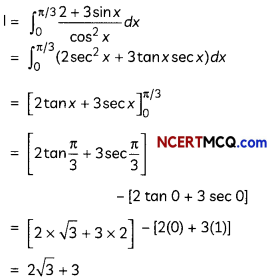
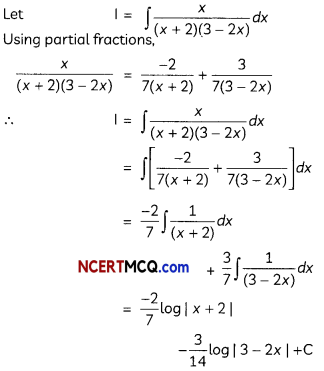
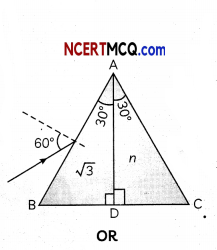
(A) Given below is the picture of the plant Rauwolfia vomitoria. Genetic variation is very important in this plant. Why?

(B) A bird named Great Indian Bustard found in Rajasthan is a threatened species. What do you mean by threatened species?
OR
Rahul read an article that hunting and human population growth is major threats to the Bengal tigers. Due to these reasons, it has become an endangered species. What do you mean by endangered species? Give two examples. (2)
![]()
Question 3.
(A) Name the gases that are produced in anaerobic sludge digesters.
(B) Which enzyme is used as a clot-buster in patients who have undergone myocardial infarction, to remove clots from blood vessels? (2)
Question 4.
Some individuals experience sudden sneezing and wheezing after reaching a hill station, while these symptoms disappear when they return to the plains. What is such a response called? How does the body produce it? (2)
Question 5.
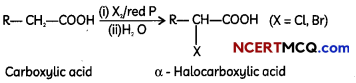
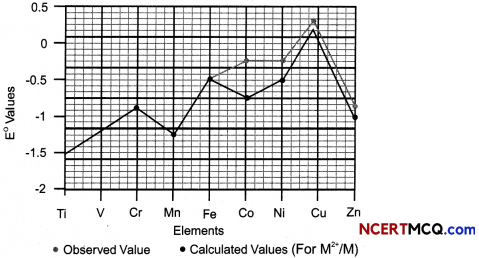
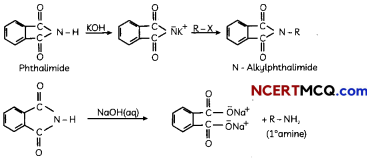
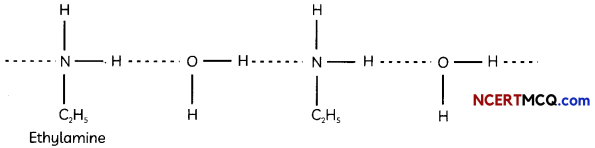
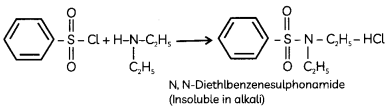
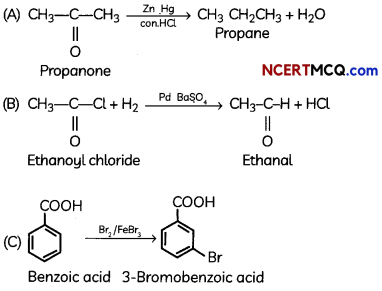
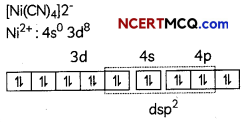
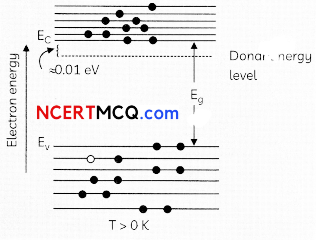
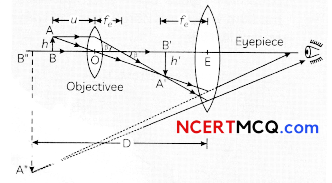
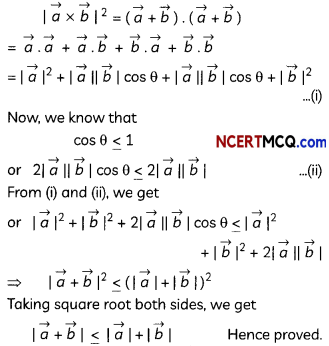
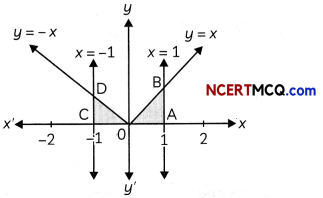
Egrets and grazing cattle are often seen together as depicted in the picture. What is this type of interaction called? Give a reason for such kind of association.

OR
Illustrate the relationship between biotic potential and environmental resistance. (2)
Question 6.
(A) Define Antibiotics.
(B) Which was the first antibiotic and who discovered it? (2)
SECTION – B
(Section B has 6 Questions of 3 marks each.)
Question 7.
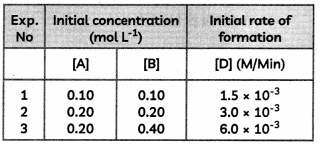
(A) If one of the insect pollinators becomes extinct in an ecosystem. Would it affect the ecosystem? If yes, how?
(B) Seed banks are used to store seeds of different genetic strains. Briefly describe the conservative strategy involved in this.
(C) From the study of the fossil records, it was found that mass extinction of species happened earlier, even before humans appeared. What could have triggered mass extinctions of species in the past? (3)
![]()
Question 8.
(A) Why malaria is restricted to the tropics?
(B) Explain why tetanus antitoxin is given to a person injured in an accident with a bleeding wound and not a tetanus vaccine? (3)
Question 9.
Explain briefly how desert ephemeral plants are adapted to withstand hot and dry environments?
OR
(A) How would you classify organisms on the basis of their tolerance to salinity?
(B) Certain animals like fungi, zooplankton and bears have the ability to cope up with the temporary short-lived stressful environmental conditions. How do they do it? (3)
Question 10.
(A) Reema (who works in a genetic engineering firm) was making her friend understand that manipulation of living organisms by the human race is not ethical and should be regulated. Is it true? Why? Name the set of standards which are used to regulate human activities in relation to biological world.
(B) A human protein a-1 antitrypsin is produced in a transgenic animal created by the introduction of the gene which codes for it. For what purpose this protein is used? (3)
Question 11.
(A) In most of the genetic engineering experiments, mice are used as the preferred organism for transgenic production. Why?
(B) RNA interference involves the silencing of specific mRNA that helps to prevent many parasitic infestations. How? (3)
Question 12.
(A) What are the different places where methanogens are found?
(B) How do methanogens help in producing biogas? (3)
SECTION – C
(Section C has a case-based question of 5 marks.)
Question 13.
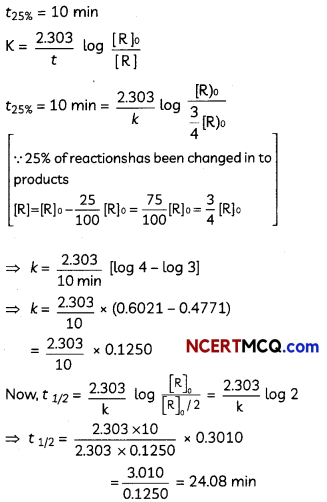
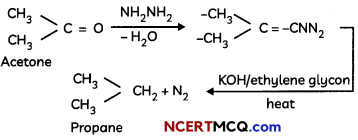
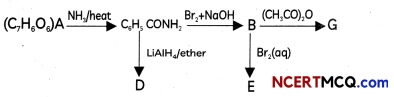
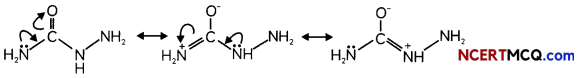
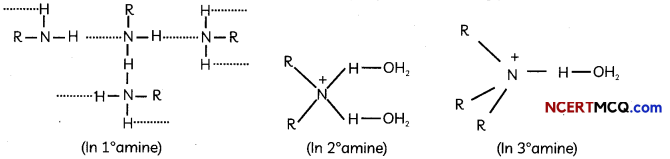
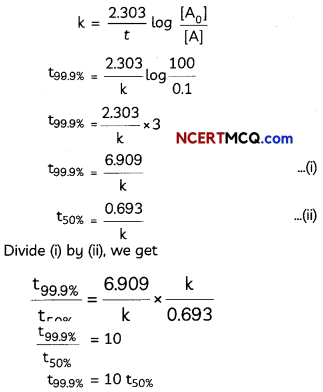
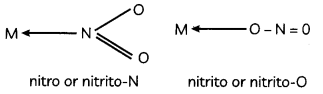
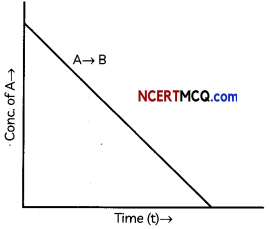
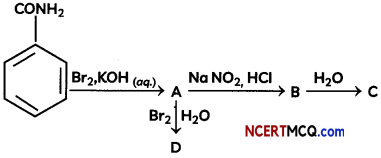
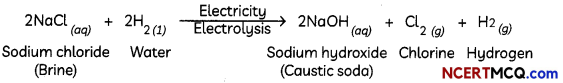
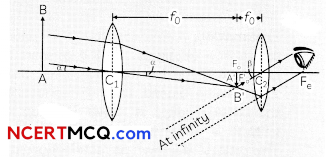
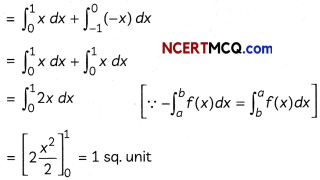
During o visit to a genetic engineering laboratory, students saw a PCR machine as shown in the picture. The students were amazed os their teacher told them, this small machine can make miLlions of copies of DNA from a very small sample of blood, hair, etc, and that too within few hours. Based on this answer the following questions:

(A) Who deveLoped the technique of PCR?
(B) What ¡s the basis of the technique of PCR?
(C) What ¡s the source of Tag Potymerose enzyme used in PCR?
(D) Name the finaL step of PCR and explain it briefly.
![]()
OR
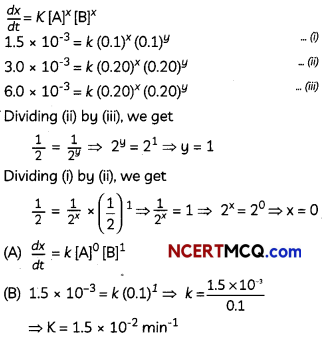
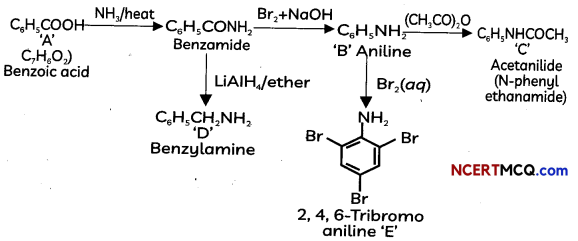
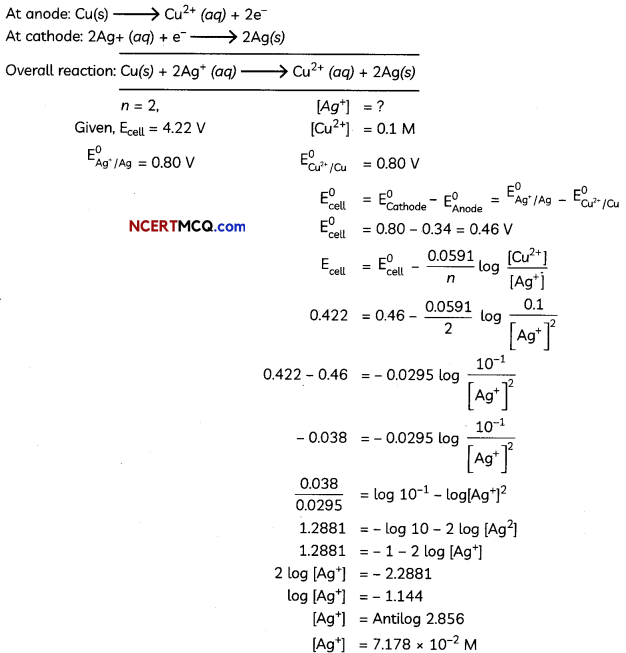
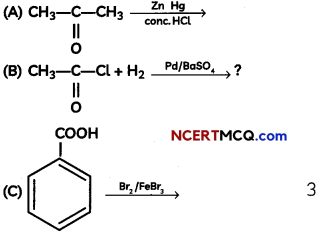
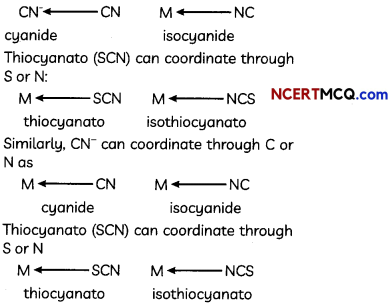
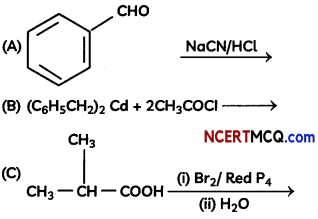
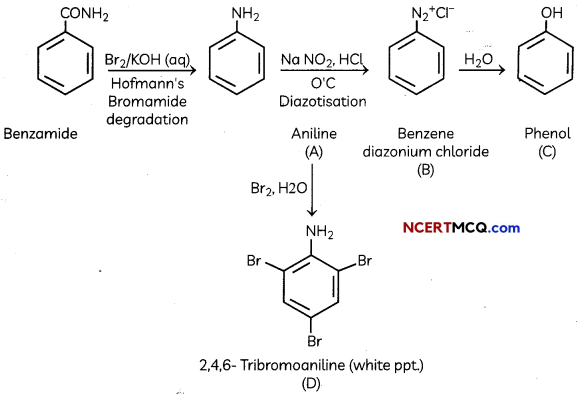
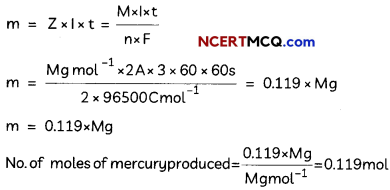
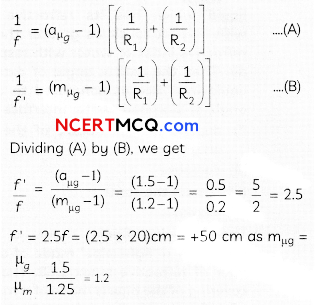
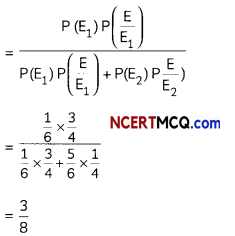
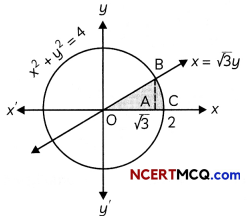
The following picture shows different types of bioreactors that are used in downstream processing.
Based on the below picture answer the following questions:
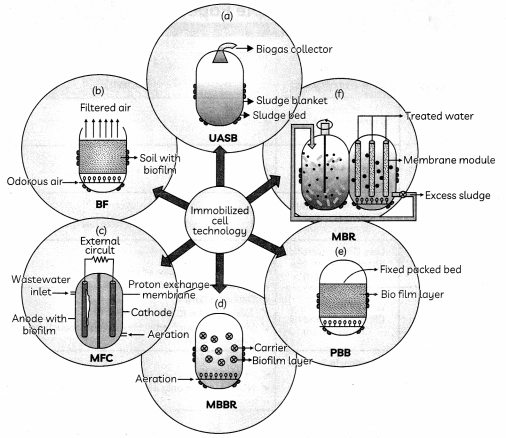
(A)
(i) Why mechanical agitation is required in stirred tank bioreactors and not in air-lift reactors?
(ii) What do you mean by continuous culture? List one of its advantages.
(B) List any two advantages of stirred tank bioreactors over shake flasks. (5)