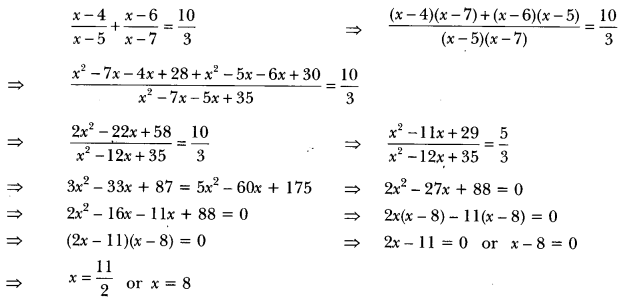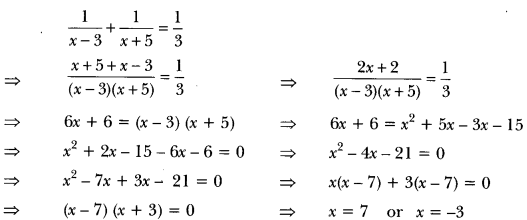In this page, we are providing Life Processes Class 10 Extra Questions and Answers Science Chapter 6 pdf download. NCERT Extra Questions for Class 10 Science Chapter 6 Life Processes with Answers will help to score more marks in your CBSE Board Exams. https://ncertmcq.com/extra-questions-for-class-10-science/
Class 10 Science Chapter 6 Extra Questions and Answers Life Processes
Extra Questions for Class 10 Science Chapter 6 Life Processes with Answers Solutions
Extra Questions for Class 10 Science Chapter 6 Very Short Answer Type
Life Processes Class 10 Extra Questions Question 1.
What will happen to a plant if its xylem is removed? [CBSE 2009]
Answer:
Xylem helps in the transport of water and minerals to the various parts of the plant. If xylem is removed it would ultimately lead to the death of the plant.
Life Processes Class 10 Extra Questions With Answers Question 2.
Name the green dot like structures in some cells observed by a student when a leaf peel was viewed under a microscope. What is the green colour due to? [CBSE 2010]
Answer:
The green dots like structures seen are the chloroplasts. The green colour is due to the pigment called chlorophyll.
Life Processes Extra Questions Question 3.
Give one reason why multicellular organisms require special organs for exchange of gases between their body and their environment. [CBSE 2010]
Answer:
Simple diffusion is not sufficient for the exchange of gases in multicellular organisms as all their cells are not in direct contact with the environment. So, they require special organs for exchange of gases between their body and their environment.
Extra Questions Of Life Processes Class 10 Question 4.
What process in plants is known as transpiration? [CBSE 2008]
Answer:
The loss of water in the form of vapour from the aerial parts of the plant is known as transpiration.
Class 10 Science Chapter 6 Extra Questions Question 5.
What is osmoregulation? [CBSE 2006]
Answer:
The maintenance of optimum concentration of water and salts (electrolytes) in the body fluids is called as osmoregulation.
Life Process Extra Question Answer Question 6.
Why is carbon dioxide mostly transported in dissolved form?
Answer:
Carbon dioxide is mostly transported in the dissolved form as it is more soluble in water.
Life Processes Class 10 Extra Questions Pdf Question 7.
When we breathe out, why does the air passage not collapse? [CBSE 2014]
Answer:
Rings of cartilage present on trachea prevent it from collapsing during the passage of air.
Class 10 Life Processes Extra Questions Question 8.
Herbivores have longer small intestine while carnivores have shorter small intestine. Give reason. [CBSE 2014]
Answer:
Herbivores have a longer small intestine compared to the carnivores to allow time for the cellulose present in the grass to get digested.
Extra Questions On Life Processes Class 10 Question 9.
Mention the respiratory unit of lungs and excretory unit of kidneys. [CBSE 2014]
Answer:
The respiratory unit of lungs are alveoli and excretory unit of kidneys are nephrons.
Class 10 Science Ch 6 Extra Questions Question 10.
Some organisms derive nutrition from plants or animals without killing them. What are these organisms called? Write one example. [CBSE 2014]
Answer:
They are called parasites, e.g. Cuscuta and tapeworm are the parasites of plants and animals respectively.
Important Questions For Class 10 Science Chapter 6 Life Processes Question 11.
What are the major constituents of urine? [CBSE 2008]
Answer:
Urine is an aqueous solution of water, urea, chloride, sodium, potassium, creatinine and other dissolved ions, inorganic and organic compounds (proteins, hormones, and metabolites).
Chapter 6 Science Class 10 Extra Questions Question 12.
Where does the urine produced by the kidneys get stored? [CBSE 2006]
Answer:
Urinary bladder.
Extra Questions Life Processes Class 10 Question 13.
How does transpiration help in upward transport of substances? [CBSE 2008]
Answer:
Transpiration creates a suction pressure which pulls up water along with the minerals through the xylem.
Life Processes Class 10 Extra Questions And Answers Question 14.
When the right atrium contract, blood flows from it to which part of the heart? [CBSE 2007]
Answer:
Right ventricle.
Ch 6 Science Class 10 Extra Question Question 15.
State the functions of the following:
(i) Blood
(ii) WBC [CBSE 2011, 2012]
Answer:
Function of RBC – To carry oxygen to various parts of the body.
Function of WBC – To protect the body against both infectious diseases and foreign invaders.
Extra Questions For Class 10 Science Chapter 6 Question 16.
Write the functions of the two upper chambers of the human heart. [CBSE 2011]
Answer:
The right atrium receives deoxygenated blood from various parts of the body through the vena cava.
The left atrium receives the oxygenated blood from the lungs through the pulmonary artery.
Ncert Class 10 Science Chapter 6 Extra Questions Question 17.
Leakage of blood from vessels reduces the efficiency of pumping system. How is the leakage prevented? [CBSE 2010, 2011]
Answer:
Leakage is prevented by the blood platelets present in the blood which help in clotting the blood at the site of injury.
Class 10 Life Process Extra Questions Question 18.
Which mechanism plays an important role in transportation of water in plants
(i) During daytime
(ii) At night? [CBSE 2011, 2012]
Answer:
During daytime – Transpiration; At Night – Root pressure.
Extra Questions Of Chapter 6 Science Class 10 Question 19.
Why are valves present in heart and the veins? [CBSE 2010,2011]
Answer:
Valves present in the heart does not allow the blood to flow backwards when the atria or ventricles contracts. Valves are present in the veins to prevent the back flow of blood in the veins as it travels at very slow rate in the veins.
Extra Questions for Class 10 Science Chapter 6 Short Answer Type I
Class 10 Science Life Process Extra Questions Question 1.
How are the fats digested in our bodies? Where does this process take place? [CBSE 2011]
Answer:
Bile juice produced by the liver breaks down the large fat globules into smaller globules by the process of emulsification. These small globules are then digested by the fat digesting enzymes. This process takes place in the small intestine.
Extra Questions From Life Processes Class 10 Question 2.
State the function of the epiglottis. [CBSE 2004]
Answer:
Epiglottis covers the opening of the wind pipe (the glottis) and prevents the entry of food into the wind pipe during swallowing.
Extra Questions For Class 10 Science Life Processes Question 3.
The breathing cycle is rhythmic, whereas exchange of gases is a continuous process. Comment upon this statement.
Answer:
Some volume of air called as residual volume is left behind in the lungs even after forceful breathing out of air. This helps to provide sufficient time for oxygen to be absorbed and for carbon dioxide to be released. Even in the absence of continuous breathing, the exchange of these gases is continuous. Hence, breathing cycle is rhythmic, whereas exchange of gases is a continuous process.
Class 10 Chapter 6 Science Extra Questions Question 4.
What are the end products formed during fermentation in yeast? Under what condition a similar process takes place in our body that lead to muscle cramps? [CBSE 2010]
Answer:
The end products formed during fermentation in yeast are ethanol and carbon dioxide. A similar process occurs in the muscles and produces lactic acid during anaerobic respiration in the muscles. Accumulation of lactic acid in the muscle cells lead to muscular cramps.
Extra Questions Of Chapter Life Processes Class 10 Question 5.
Give Reasons:
(a) Rings of cartilage are present in the trachea.
(b) Lungs always contain a residual volume of air. [CBSE 2013]
Answer:
(a) The walls of trachea have rings of cartilage on them which prevent it from collapsing.
(b) The volume of air left behind in the lungs even after forceful breathing out of air is called as residual volume. This helps to provide sufficient time for oxygen to be absorbed and for the carbon dioxide to be released.
Question 6.
State in brief the role of lungs in the exchange of gases. [CBSE 2012]
Answer:
Lungs have alveoli which provide a larger surface for exchange of gases and are richly supplied with blood vessels to enable faster exchange. So, lungs help in providing oxygen to various tissues of the body and removal of carbon dioxide from the body.
Question 7.
What is the basic unit of kidney called? Why is it composed of very thin blood capillaries? [CBSE 2015]
Answer:
The basic unit of kidney is called nephron. It is composed of a cluster of very thin blood capillaries as they help in filtration of blood and remove the nitrogenous wastes from the body in the form of urine.
Question 8.
How does the plant get rid of excretory products? [CBSE 2009]
Answer:
Excess oxygen and carbon dioxide removed through stomata.
Plant waste products are also removed by:
- Storage in cellular vacuoles
- Storage in leaves that fall off
- Storing as resins and gums in old xylem
- By excreting into the soil around them.
Question 9.
Tabulate two differences between renal artery and renal vein. [CBSE 2009]
Answer:
Renal Artery
- Blood in renal artery contains glucose, oxygen and cellular waste products.
- It takes blood towards the kidney.
Renal Vein:
- Blood in renal vein is filtered, and is free from cellular waste and any other impurities.
- It takes blood away from the kidney towards the heart.
Question 10.
(a) What is the main toxic waste that kidney filters from the blood?
(b) Name any two substances which are selectively reabsorbed from the tubules of a nephron. [CBSE 2010, 2012]
Answer:
(a) Urea is the main excretory product removed by the kidneys of human beings.
(b) The substances selectively reabsorbed by the kidneys are water, glucose, electrolytes, etc.
Question 11.
What is excretion? How do unicellular organisms remove their wastes? [CBSE 2012]
Answer:
Removal of metabolic wastes from the body is called as excretion. Many unicellular organisms remove metabolic wastes from the body surface into the surrounding water by simple diffusion.
Question 12.
Write a function of (a) blood vessels (b) blood platelets. [CBSE 2008]
Answer:
(a) Blood vessels help in carrying blood to various parts of the body.
(b) Blood platelets help in the clotting of blood at the point of injury to prevent non-stop bleeding.
Question 13.
How are water and minerals absorbed by the plant? [CBSE 2010]
Answer:
The water and minerals in the soil are absorbed by plants with the help of root hairs present on their roots. Root hairs provide a larger surface area for absorption.
Question 14.
What are capillaries? Sate the function performed by them. [CBSE 2012]
Answer:
The capillaries are one-cell thick, small blood vessels which help in the exchange of materials between the blood and the surrounding tissues.
Question 15.
Mention the two main components of the transport system in plants. State one function of each one of these components. [CBSE 2010, 2011]
Answer:
The two main components of the transport system in the plants are xylem and phloem. Xylem helps to transport water and minerals to various parts of the plant. Phloem helps to carry food from leaves to the various parts of the plant.
Question 16.
During one cycle how many times does blood go to the heart of fish and why? [CBSE 2010]
Answer:
The blood passes only once through the heart in one cycle in fishes because the two-chambered heart of the fishes pump the blood to gills for oxygenation. The blood from gills is then directly passed to the various parts of the body in the fishes.
Question 17.
What would be the consequences of deficiency of haemoglobin in our bodies? [CBSE 2012]
Answer:
Haemoglobin helps in transport of oxygen to the body parts. Deficiency of haemoglobin will affect transport of oxygen and the person will suffer from improper metabolism, weakness, fatigue and pain.
Question 18.
Name the following:
(а) The process in plants that links light energy with chemical energy.
(b) Organisms that can prepare their own food.
(c) The cell organelle where photosynthesis occurs.
(d) Cells that surround a stomatal pore.
(e) Organisms that cannot prepare their own food.
(f) An enzyme secreted from gastric glands in stomach that acts on proteins. [NCERT Exemplar]
Answer:
(a) Photosynthesis
(b) Autotrophs
(c) Chloroplast
(d) Guard cells
(e) Heterotrophs
(f) Pepsin
Question 19.
“All plants give out oxygen during the day and carbon dioxide during night”. Do you agree with this statement? Give reason. [NCERT Exemplar]
Answer:
The rate of photosynthesis is higher than the rate of respiration during the daytime, so the net result is the evolution of oxygen. In the absence of photosynthesis at night, only respiration occurs in the plants so carbon dioxide is released at night.
Question 20.
How do the guard cells regulate opening and closing of stomatal pores? [NCERT Exemplar]
Answer:
The entry of water into the guard cells of the stomata causes an increase in turgor pressure in the guard cells which leads to opening of the stomata. The loss of water from the guard cells results in their shrinking and closes the stomata.
Question 21.
Two green plants are kept separately in oxygen free containers, one in the dark and the other in continuous light. Which one will live longer? Give reasons. [NCERT Exemplar]
Answer:
Plant kept in continuous light will perform photosynthesis and release oxygen for its respiration. Hence, it will live longer than the plant kept in the dark.
Question 22.
If a plant is releasing carbon dioxide and taking in oxygen during the day, does it mean that there is no photosynthesis occurring? Justify your answer. [NCERT Exemplar]
Answer:
During the day time the plants take in carbon dioxide and release oxygen as a by product of photosynthesis. Release of carbon dioxide and taking in air during the daytime means that either the rate of photosynthesis is too low or its not occurring at all.
Question 23.
Why do fishes die when taken out of water? [NCERT Exemplar]
Answer:
Fishes take water from mouth and send it to the gills which are richly supplied with blood capillaries for absorbing the oxygen dissolved in water. But the fishes cannot absorb gaseous oxygen, so they die soon after they are taken out of water.
Question 24.
Is ‘nutrition’ a necessity for an organism? Discuss. [NCERT Exemplar]
Answer:
Nutrition (food) is a necessity for an organism as
- It provides energy for the various metabolic processes in the body.
- It is essential for the growth and repair of various cells and tissues.
- It helps to provide resistance against various diseases.
Question 25.
What would happen if green plants disappear from the Earth? [NCERT Exemplar]
Answer:
The green plants are the source of energy for the entire organisms on the Earth. Herbivores depend directly on the plants while the carnivores and omnivores depend either directly or indirectly on plants. So, all the organisms will die due to starvation if all the green plants disappear from the Earth.
Question 26.
Leaves of a healthy potted plant were coated with vaseline. Will this plant remain healthy for long? Give reasons for your answer. [NCERT Exemplar]
Answer:
This plant will not remain healthy for a long time because the stomata will get blocked, so plant
- Will not get carbon dioxide for photosynthesis.
- Will not get oxygen for respiration.
- Will not be able to do transpiration which will in turn affect the upward transport of water and minerals.
Extra Questions for Class 10 Science Chapter 6 Short Answer Type II
Question 1.
(a) Explain why the rate of photosynthesis is low both at lower and higher temperatures.
(b) Is green light most or least useful in photosynthesis and why? [CBSE 2005]
Answer:
(a) The reactions occurring during the process of photosynthesis is under the control of enzymes, which work under an optimum range of temperature only. The very high temperatures as well as very low temperatures decrease the activity of the enzymes. So, the rate of photosynthesis is low both at lower and higher temperatures.
(b) Chlorophyll does not absorb the green light, so the green light is least useful in photosynthesis.
Question 2.
Draw a well labelled diagram of stomata. List two functions of stomata. [CBSE 2011]
Answer:
The function of stomata are:
(a) Help in the exchange of gases like carbon dioxide and oxygen from the leaves of the plants.
(b) Help in the transport of water, minerals and food materials in plants by transpiration.
(c) Transpiration occurring through stomata on leaves helps in cooling of leaf surface.
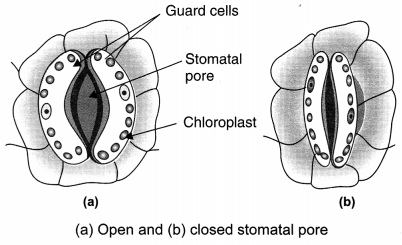
Question 3.
Explain how the products of photosynthesis and other substances are translocated in plants? [CBSE 2015]
Answer:
Translocation is the transport of soluble products of photosynthesis through phloem. Sucrose is transferred into sieve tubes of phloem via the companion cells using energy from ATP. This increases the osmotic pressure inside the sieve tubes which causes movement of water into the sieve tubes from the adjacent xylem. This pressure helps in translocation of material in the phloem to tissues which have less pressure.
Question 4.
Write three events which occur during the process of photosynthesis. [CBSE 2015]
Answer:
The three events which occur during photosynthesis are:
- Absorption of light energy of chlorophyll.
- Conversion of light energy to chemical energy + splitting of water molecules into hydrogen and oxygen.
- Reduction of carbon dioxide to carbohydrates.
Question 5.
Explain why the transportation of materials is necessary in animals? [CBSE 2014]
Answer:
The transportation system is necessary to transport the various nutrients and gases to and from the various parts of the body. It also helps in removing the wastes from the body of the organisms. The various life processes are maintained and carried out due to an efficient transport system in animals.
Question 6.
Plants absorb water from the soil. Explain how does the water reach the tree top? [CBSE 2014]
Answer:
There are two ways for the transport of water in plants:
(а) By root pressure: The cells of root in contact with soil actively take up ions which creates a difference in ion concentration between the root and the soil. Water moves into the root from the soil to eliminate this difference, creating a column of water that is steadily pushed upwards.
(b) By transpiration pull: Loss of water from stomata by transpiration gets replaced by the xylem vessels in the leaf which creates a suction to pull water from the xylem cells of the roots. This strategy is used during day time and helps to transport water to the highest points of the plant body.
Question 7.
State the function of the following in the alimentary canal.
(a) Liver
(b) Gallbladder
(c) Villi [CBSE2014]
Answer:
(a) Liver: Helps in detoxification of harmful chemicals. Produces bile juice which helps in digestion of fats.
(b) Gall bladder: Helps in the storage of bile juice released from the liver.
(c) Villi: Helps to increase the surface area of the small intestine and aid in absorption of the digested nutrients.
Question 8.
(a) How does exchange of respiratory gases—oxygen and carbon dioxide take place between tissues and blood in human beings?
(b) Name the respiratory pigment in humans. Where is it found? [CBSE 2014]
Answer:
(a) The arteries have oxygenated blood having oxygen at higher pressure to that present in the tissues. Carbon dioxide is present at a higher pressure inside the tissues. The oxygen is thus exchanged at the tissue surface with carbon dioxide in order to supply oxygen to the tissues of the body.
(b) Haemoglobin is the respiratory pigment in humans which is present in the red blood cells.
Question 9.
Explain giving any three reasons the significance of transpiration in plants. [CBSE 2014]
Answer:
The significance of transpiration is:
- Absorption and upward movement of water and minerals.
- It also helps in temperature regulation by cooling the leaf surface.
- It helps to maintain the shape and size of the cells.
Question 10.
Mention the pathway of urine starting from the organ of its formation. Name four substances which are reabsorbed from the initial filtrate in the tubular part of the nephron. [CBSE 2014]
Answer:
The urine formed in the kidney moves through the ureters to the urinary bladder. Ureters takes urine into the urinary bladder where it is stored until it is released through the urethra. Release of urine is under nervous control.
Glucose, amino acids, salts and water are reabsorbed from the initial filtrate in the tubular part of the nephron.
Question 11.
Major amount of water is selectively reabsorbed by the tubular part of nephron. On what factor does the amount of water reabsorbed depends? [CBSE 2010, 2011, 2012]
Answer:
The amount of water reabsorbed by the tubular part of nephron depends on two factors:
- The amount of nitrogenous and other excretory wastes present in the body.
- The amount of excess water present in the body.
Question 12.
State the functions of
(a) Renal artery
(b) kidney
(c) urinary bladder. [CBSE 2012]
Answer:
(a) Renal artery: It brings blood having nitrogenous wastes to nephrons for filtration.
(b) Kidney: It help to filter the nitrogenous and other wastes from the blood.
(c) Urinary bladder: Stores urine until it is eliminated.
Question 13.
(a) Write the important functions of the structural and functional unit of kidney.
(b) Write any one function of an artificial kidney. [CBSE 2011]
Answer:
(a) The structural and functional units of kidney are the nephrons which help in filtration, reabsorption and secretion. They help to eliminate the wastes from the body.
(b) Artificial kidneys help to remove the harmful nitrogenous wastes from the body of a patient whose kidneys are not functioning properly.
Question 14.
How is urine produced? [CBSE 2011]
Answer:
The urine formation involves three steps:
(a) Glomerular filtration: Nitrogenous wastes, glucose water, amino acid are filtered from the blood in blood capillaries into Bowman Capsule of the nephrons.
(b) Selective reasbsorption: Some substances in the initial filtrate, such as glucose, amino acids, salts and a major amount of water are selectively reabsorbed back by capillaries surrounding the nephrons.
(c) Tubular secretion: Some ions like K+, H+, etc. are secreted into the tubule which opens up into the collecting duct.
Question 15.
Blood does not clot in the blood vessels. Give reasons. [CBSE 2009]
Answer:
Blood does not clot inside the blood vessels because the chemical which causes the blood to clot in the cut area gets activated only when it comes in contact with air (oxygen actually) to form the clotting substance. The chemical for clotting of blood is released by the blood platelets.
Question 16.
What is translocation? How does it take place in plants? [CBSE 2011]
Answer:
Translocation is the movement of materials from leaves to other tissues throughout the plant by the phloem. During translocation, sucrose is transferred into sieve tubes of phloem via the companion cells using energy from ATP. This increases the osmotic pressure inside the sieve tubes which causes movement of water into the sieve tubes from the adjacent xylem. This pressure helps in translocation of material in the phloem to tissues which have less pressure.
Question 17.
(a) Transport of food in plants require living tissues and energy. Justify this statement.
(b) Name the components of food that are transported by the living tissues. [CBSE 2012]
Answer:
(a) The energy in the form of ATP is required both during loading of sucrose in the sieve tubes at the leaf surface and during uploading of the sucrose at the site which needs sucrose for its metabolic activities. So, it can be said that transport of food in plants require living tissues i.e., phloem and energy in the form of ATP.
(b) The components of food transported by the phloem are sugars, hormones, and minerals elements dissolved in water.
Question 18.
What are the adaptations of leaf for photosynthesis? [NCERT Exemplar]
Answer:
The various adaptations in leaf for photosynthesis are:
- Leaves have expanded portion to provide large surface area for maximum light absorption.
- Leaves are arranged at right angles to the light source in a way that causes overlapping.
- The extensive network of veins helps in quick transport of substances in leaf.
- Easy gaseous exchange is facilitated by the presence of stomata on the leaves.
- The chloroplasts are more in number on the upper surface of leaves.
Question 19.
Why is small intestine in herbivores longer than in carnivores? [NCERT Exemplar]
Answer:
Herbivores have a longer small intestine compared to the carnivores to allow time for the cellulose present in the grass to get digested. Cellulose takes longer time to get digested. Animals have shorter small intestine as they are not able to digest cellulose.
Question 20.
What will happen if mucus is not secreted by the gastric glands? [NCERT Exemplar]
Answer:
Mucus forms the lining of the stomach and protects it from the action of hydrochloric acid released by the gastric glands of stomach. If mucus is not secreted then acidity and ulcers may be caused due to erosion of the inner lining of the stomach by the acid.
Question 21.
What is the significance of emulsification of fats? [NCERT Exemplar]
Answer:
The large globules of fats are broken down into smaller fat globules by the action of bile salts present in the bile juice. This is called emulsification and it increases the efficiency of the fat digesting enzymes that can digest the smaller fat globules.
Question 22.
Why does absorption of digested food occur mainly in the small intestine? [NCERT Exemplar]
Answer:
Maximum absorption occurs in small intestine because
- The process of digestion gets completed in the small intestine by intestinal juice.
- Finger-like projections called villi provide larger surface area for absorption.
- The villi are richly supplied with blood vessels to enable easy absorption into the bloodstream.
Question 23.
| Group (A) | Group (B) |
| (a) Autotrophic nutrition | (i) Leech |
| (b) Heterotrophic nutrition | (ii) Paramecium |
| (c) Parasitic nutrition | (iii) Deer |
| (d) Digestion in food vaceolus | (iv) Green plants |
Answer:
(a) (iv)
(b) (iii)
(c) (i)
(d) (ii)
Question 24.
Why is the rate of breathing in aquatic organisms much faster than in terrestrial organisms? [NCERT Exemplar]
Answer:
Aquatic animals use the oxygen dissolved in water. They breathe at a faster rate since the amount of dissolved oxygen is fairly low compared to the amount of oxygen in the air.
Question 25.
In each of the following situations what happens to the rate of photosynthesis? [NCERT Exemplar]
(a) Cloudy days
(b) No rainfall in the area
(c) Good manuring in the area
(d) Stomata get blocked due to dust
Answer:
(a) Decreases
(b) Decreases
(c) Increases
(d) Decreases
Question 26.
Name the energy currency in the living organisms. When and where is it produced? [NCERT Exemplar]
Answer:
The energy currency in the cells is Adenosine triphosphate (ATP). It is produced in mitochondria during respiration and in chloroplasts during photosynthesis in plants.
Question 27.
What is common for Cuscuta, ticks and leeches? [NCERT Exemplar]
Answer:
Cuscuta, ticks and leeches are parasites which obtain their nutrition from plants and animals without killing them.
Question 28.
Explain the role of mouth in digestion of food. [NCERT Exemplar]
Answer:
The mouth plays an important role in digestion because
- The teeth present in the mouth crush the food into small pieces.
- Tongue helps in thorough mixing of food with saliva and to swallow the food.
- Saliva secreted in mouth contains an enzyme called salivary amylase which helps to break down the starch into maltose.
Question 29.
What are the functions of gastric glands present in the wall of the stomach?
Answer:
The functions of gastric glands present in the wall of the stomach are:
- Produce pepsin enzyme that digests proteins.
- Secrete mucus which protects the inner lining of stomach from the action of acids.
Extra Questions for Class 10 Science Chapter 6 Long Answer Type
Question 1.
How is oxygen and carbon dioxide transported in the human beings? [CBSE All India 2008]
Answer:
At the alveolar surface, the oxygen diffuses out from the alveoli to the capillaries which surround the alveoli. The oxygenated blood is transported from the lungs to the heart which pumps it to the various parts of the body to supply it to various tissues.
At the tissue surface, the oxygen from the blood diffuses out into the tissues and carbon dioxide which is at higher concentration in the tissues, diffuses out into the capillaries. The capillaries transport the carbon dioxide rich blood from tissues to the alveoli where carbon dioxide diffuses out into the alveoli and oxygen enters into the capillaries to be transported to the tissues.
Question 2.
(a) State reason for the following:
(i) Herbivores need a longer small intestine while carnivores have shorter small intestine.
(ii) The lungs are designed in human beings to maximise the area for exchange of gases.
(b) The rate of breathing in aquatic organisms is much faster than that seen in terrestrial organisms. [CBSE 2014]
Answer:
(a) (i) The cellulose present in the grasses takes a longer time to digest. So, the herbivores have a longer small intestine than the carnivores.
(ii) The alveoli present in the lungs provide a larger surface area for the exchange of gases in the lungs.
(b) Aquatic organisms breathe at a faster rate since the amount of dissolved oxygen is fairly low compared to the amount of oxygen in the air.
Question 3.
Draw a diagram of excretory unit of human kidney and label the following:
(a) Bowman’s capsule
(b) Glomerulus
(c) Collecting duct
(d) Renal artery [CBSE 2011, 2012]
Answer:
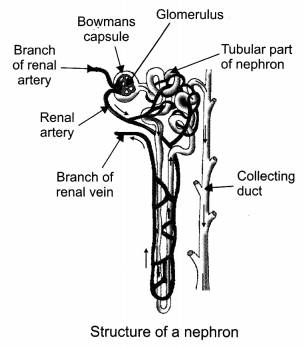
Question 4.
Explain how deoxygenated blood travels from body to lung for purification. Draw well labelled diagram in support of your answer. [CBSE 2011]
Answer:
The deoxygenated blood from the various parts of the body is collected by the veins which transport the blood to the heart through the vena cava. Vena cava pours the deoxygenated blood in the right atrium of the heart. The right atrium contracts and the blood moves into the right ventricle. On contraction of the right ventricle the deoxygenated blood is transported to the lungs through the pulmonary artery for purification.
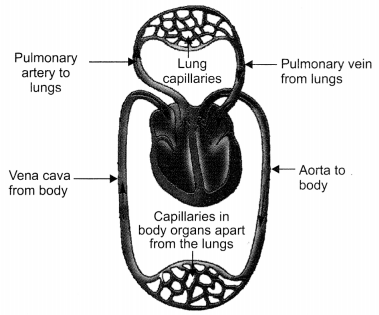
Schematic representation of transport and exchange of oxygen and carbon dioxide
Question 5.
Draw the sectional view of human heart and label the following parts given below:
(a) Chamber where oxygenated blood from lungs is collected
(b) Largest blood vessel in the body
(c) Muscular wall separating right and left chambers
(d) Blood vessel that carries blood from heart to the lungs [CBSE 2010]
OR
(a) Part which receives deoxygenated blood from vena cava
(b) Part which sends deoxygenated blood to lung through pulmonary artery
(c) Part which receives oxygenated blood from lungs
(d) Part which sends oxygenated blood to all parts of the body through aorta [CBSE 2012]
OR
(a) the chamber of heart that pumps out deoxygenated blood
(b) the blood vessel that carries away oxygenated blood from the heart
(c) the blood vessel that receives deoxygenated blood from the lower part of our body [CBSE 2010, 2011, 2012]
Answer:
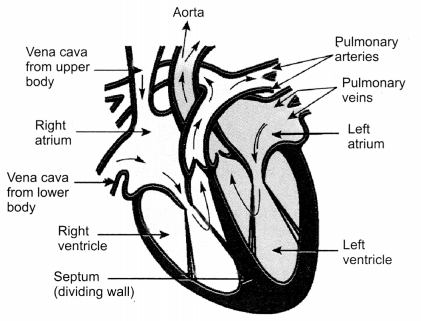
(а) Chamber where oxygenated blood from lungs is collected – Left atrium
(b) Largest blood vessel in the body – Aorta
(c) Muscular wall separating right and left chambers – Septum
(d) Blood vessel that carries blood from heart to the lungs – Pulmonary artery
OR
(а) Part which receives deoxygenated blood from vena cava – Right atrium
(b) Part which sends deoxygenated blood to lung through pulmonary artery – Right Ventricle
(c) Part which receives oxygenated blood from lungs – Left atrium
(d) Part which sends oxygenated blood to all parts of the body through aorta – Left Ventricle
OR
(а) the chamber of heart that pumps out deoxygenated blood – Right Ventricle
(b) the blood vessel that carries away oxygenated blood from the heart – Aorta
(c) the blood vessel that receives deoxygenated blood from the lower part of our body – Inferior Vena cava
Question 6.
Explain the process of nutrition in Amoeba. [NCERT Exemplar]
Answer:
Nutrition in Amoeba:
Temporary finger-like extensions of the cell surface called pseudopodia are used by Amoeba to engulf food. Pseudopodia fuse over the food particle forming a food-vacuole in which complex substances are broken down into simpler ones and diffuse into the cytoplasm. The remaining undigested material moves to the surface of the cell and gets thrown out.
Question 7.
Describe the alimentary canal of man. [NCERT Exemplar]
Answer:
Mouth: Helps in intake of whole food.
Teeth: Helps in chewing and grinding of food.
Tongue: Helps in tasting food + rolling food + swallowing food.
Salivary glands: Secrete saliva and mucus. The enzyme called salivary amylase is present in saliva which breaks down the complex starch into sugar.
Oesophagus (food pipe): Food moves towards stomach through oesophagus by rhythmic contraction of its muscles called as peristaltic movements or peristalsis.
Stomach: Muscular walls of stomach help in mixing food thoroughly with digestive juices. Stomach has gastric glands which secrete gastric juice containing pepsin for digestion of proteins, hydrochloric acid for creating acidic medium and mucus to protect inner lining of stomach from acid.
Small Intestine: Digestive juices like pancreatic juice, bile juice and the intestinal juices are secreted in the small intestine to help to complete the process of digestion. After digestion, the nutrients are absorbed by the villi present in the walls of the small intestine.
Large intestine: The unabsorbed food is sent into the large intestine where more villi absorb water from this material and remove the wastes through the anus by egestion. The exit of this waste material is regulated by the anal sphincter.
Question 8.
Explain the process of breathing in man. [NCERT Exemplar]
Answer:
Air enters the body after getting filtered by fine hairs and mucus in the nostrils.
The air then passes through trachea (present in throat) into the lungs. The trachea divide into bronchi which enter the lungs and divide further into bronchioles which finally terminate in balloon-like structures called alveoli which have a rich supply of blood vessels and help in exchange of gases.
During inhalation (breathing in), the volume of the chest cavity becomes larger as the ribs get lifted and diaphragm gets flattened. Air gets sucked into the lungs and fills the expanded alveoli. The blood brings carbon dioxide from the rest of the body to the alveoli and exchanges it for oxygen to be transported to all the cells in the body.
During exhalation (breathing out), the volume of the chest cavity becomes smaller as the ribs get relaxed and diaphragm moves upward (relaxes). Air rich in carbon dioxide gets pushed out of the lungs to come out through the nostrils.
Question 9.
Explain the importance of soil for plant growth.
Answer:
Soil is important for the plant growth as it helps in the
- Anchoring the plant.
- Acts as a source of water and minerals for the plants.
- Ensures availability of oxygen for respiration of root cells.
- Microbes living in symbiotic association are found in the soil which helps to provide nitrogen for the plants.
Life Processes HOTS Questions With Answers
Question 1.
Which part of visible the spectrum is mostly ineffective in the process of photosynthesis? Why?
Answer:
The green part of the visible spectrum is mostly ineffective in the process of photosynthesis because chlorophyll involved in the process does not absorb green light. The green component of the visible spectrum gets reflected by the chlorophyll due to which the leaves appear green.
Question 2.
What difference would be seen in the small intestine of a grass eating animal and a flesh eating animal?
Answer:
The grass eating animal would have a longer small intestine than a flesh eating animal as the cellulose present in the grass takes a longer time to get digested.
Question 3.
The gall bladder of a patient is removed when a stone was observed in the gall bladder. Which kind of nutrient in the diet should be absent from the diet given to the patient?
Answer:
The gall bladder release bile juice which helps in the digestion of fats in the human beings. So, the patient should be advised a diet free from fats during the treatment process, as the digestion of fats will be most affected due to removal of gall bladder.
Question 4.
Stomata of desert plants remain closed during the daytime. Then how do they take up carbon dioxide and perform photosynthesis? [CBSE 2010, 2012]
Answer:
The stomata of the desert plants open during the night time and absorb carbon dioxide to use it during the daytime for performing photosynthesis.
Question 5.
How do carbohydrates, proteins and fats get digested in human beings?
Answer:
An enzyme called salivary amylase is present in the saliva produced in the mouth. Salivary amylase helps in the breakdown of carbohydrates into maltose in the mouth.
The proteins get digested initially by the pepsin enzyme in the stomach and then by the enzymes present in the pancreatic juice secreted into the small intestine. The fats are broken down into small globules by the bile salts present in the bile juice secreted into the small intestine. This helps in increasing the efficiency of fat digesting enzymes.
The intestinal juice secreted by the walls of the small intestine help to complete the digestion of carbohydrates into glucose/monosaccharide, digestion of proteins into amino acids and the digestion of fats into fatty acids and glycerol.
Extra Questions for Class 10 Science Chapter 6 Value Based Questions
Question 1.
Raksha planted many plants in her home garden as she knew that the plants help to purify air. To get purified air in her room she kept some of the plants in a closed room, it leaves turned pale in colour after some days.
(a) Define photosynthesis. What are the conditions necessary for photosynthesis?
(b) What are the values shown by Raksha?
(c) What can be the most probable reason which resulted in the pale leaves?
Answer:
(a) The process by which the autotrophs synthesise their own food is called as photosynthesis.
Photosynthesis,occurs in the presence of carbon dioxide, water, chlorophyll and sunlight.
(b) The values shown by Raksha are intelligence, environment friendly, scientific temper, creative thinking.
Question 2.
Ayush experienced muscular cramps during the training session for his upcoming cricket match. His coach advised him a schedule of aerobic exercises to overcome this problem. Ayush followed his coach’s advice and did not experience any muscular cramps during the game. Based on this, answer the following questions:
(а) What was the reason for the muscular cramps?
(b) What was the effect of aerobic exercises?
(c) What are the values shown by Ayush?
Answer:
(a) The muscular cramps were caused as the muscles produced lactic acid during anaerobic respiration in the muscle cells. The accumulation of lactic acid caused the cramps.
(b) Aerobic exercises helped in providing enough oxygen for the various muscle cells so that they had a more supply of oxygen.
(c) Values shown by Ayush are respect for his coach, responsibility, hard work and patience.
Question 3.
Anshika noticed that one of the plants in her garden had wrinkled and drooping leaves. She went to the garden and felt the soil below the plant which was too dry. She immediately made provisions for watering the plant and made a daily schedule for watering it every day. Within a few days the plant revived and became green and healthy. On the basis of this text, answer the following questions:
(a) How is water transported up into the plants?
(b) What is the role of the leaves and the roots in such transport?
(c) What are the values shown by Anshika?
Answer:
(a) Water is transported up into the plants with the help of xylem. The movement of water in the xylem occurs due to transpiration and root pressure.
(b) Roots absorb water through their root hairs. Leaves have stomata through which transpiration occurs and pulls up the water through the xylem to the top of the plant.
(c) The values shown by Anshika are: Love for nature, scientific aptitude, awareness, care
Question 4.
Sameer went to a forest and observed the villagers collecting reins and gums from the plants. He asked the villagers about the use of the resins and gums. They told him that they will be used in making the paints and varnishes for furniture. He learnt the technique of collecting resins from the villagers and enjoyed the activity with the villagers by helping them in collection.
On the basis of the text, answer the following questions:
(а) Why are resins and gums released by plants?
(b) What are the ways in which plants get rid of their waste products?
(c) What are the values shown by Sameer?
Answer:
(a) Resins and gums are the excretory products of the plants.
(b) Plants remove excess oxygen and carbon dioxide through stomata, excess water by transpiration through stomata and other waste products by storage in cellular vacuoles, leaves that fall off, storage as resins and gums in old xylem or by excreting into the soil around them.
(c) Values shown by Sameer are: Care of the environment, scientific attitude, helpfulness, curiosity.