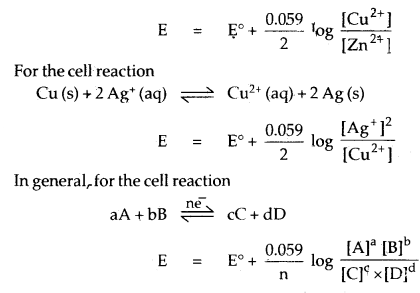
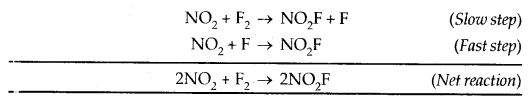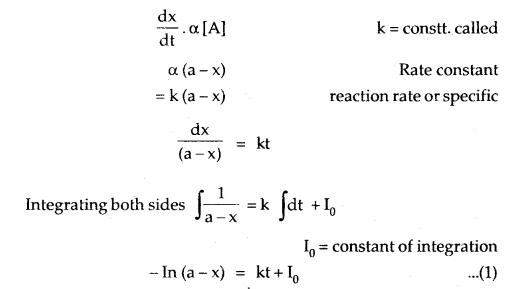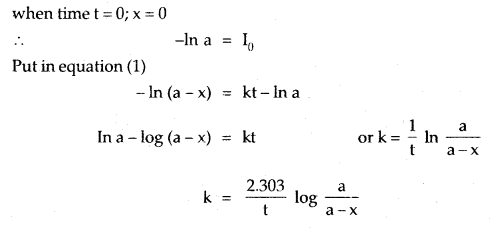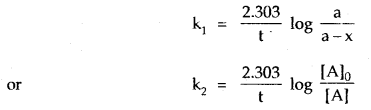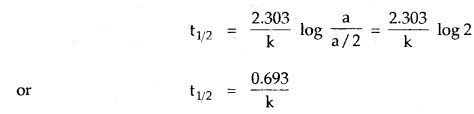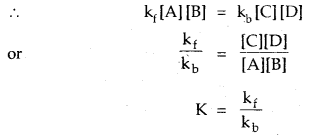By going through these CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry, students can recall all the concepts quickly.
Surface Chemistry Notes Class 12 Chemistry Chapter 5
The branch of chemistry which deals with the nature of surface and species present on it is called surface chemistry. Adsorption on solid or on solution surfaces and colloidal properties are important surface effects.
Adsorption: The phenomenon of higher concentration of molecular species (gases or liquids) on the surface of solids than in the bulk is called adsorption.
The solid on the surface of which adsorption occurs is called adsorbent. The substances that get adsorbed on the solid surface is called adsorbate. The adsorbent may be a solid or a liquid and the adsorbate may be a gas or a liquid.
The phenomenon of absorption differs from adsorption as:
| Absorption | Adsorption |
| 1. It is the phenomenon in which the particles of gas or liquid get uniformly distributed throughout the body of the solid. | 1. It is the phenomenon of higher concentration of particles of gas or liquid on the surface than if! the bulk of the solid. |
| 2. Absorption occurs at uniform rate. | 2. Adsorption is rapid in the beginning and its rate slowly decreases. |
Types Of Adsorption:
Depending upon the nature of forces between the molecules of the adsorbate and the adsorbent, the adsorption maybe classified as: physical adsorption and chemical adsorption.
1. Physical adsorption: When the particles of the adsorbate are held to the surface of the adsorbent by the weak forces such as van der Waals forces, the adsorption is called physical adsorption or physisorption. The attractive forces are weak and therefore, these can be easily overcome either by increasing the temperature or by decreasing the pressure. In other words, physical adsorption can be easily reversed or decreased.
2. Chemical adsorption: When the molecules of the adsorbate are held to the surface of the adsorbent by the chemical forces, the adsorption is called chemical adsorption or chemisorption, hi this case, a chemical reaction occurs between the adsorbed molecules and the molecules or atoms of adsorbent.
Table: Comparison between Physisorption and Chemisorption:
| Physisorption | Chemisorption |
| 1. Enthalpy of adsorption, usually is of the order of 20 – 40 kJ mol-1 | 1. Enthalpy of adsorption, is of the order 40-400 kJ mol-1. |
| 2. Molecules of adsorbate and adsorbent are held by van der Waals interactions. | 2. Molecules of adsorbate and adsorbent are held by chemical bonds. |
| 3. It usually takes place at low temperature and decreases with increasing temperature. | 3. It takes place at relatively high temperatures. |
| 4. It is not very specific i.e., all gases are absorbed on all solids to some extent. | 4. It is highly specific and takes place when there is some possibility of compound formation between the adsorbate and the adsorbent molecules. |
| 5. Multi-molecular layers may be formed on the adsorbent. | 5. Usually mono-molecular layer is formed on the adsorbqnt, |
| 6. It does not require any activation energy. | 6. It requires activation energy. |
| 7. The amount of gas adsorbed is related to the ease of liquefaction of the gas. | 7. There is no such correlation. |
| 8. it is reversible in nature. | 8. It is irreversible in nature. |
Freundlich Adsorption Isotherm:
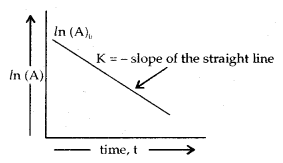
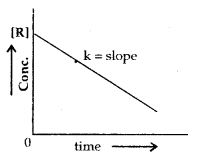
The adsorption of a gas on the surface of the solid depends upon the pressure of the gas. The extent of adsorption is generally expressed as x/m where m is the mass of the adsorbent and x is the mass of adsorbate when equilibrium has been attained. On the basis of experimental studies, Freundlich gave the following relationship between the amount of gas adsorbed (x) per unit mass of the adsorbent (m) and the pressure (p).
\(\frac{x}{m}\) = kp1/n
where n is a constant (whole number) which depends upon the nature of adsorba te and adsorbent.
A graph between the amount (x/m) adsorbed by an adsorbent and the equilibrium pressure (or concentration for solutions) of the adsorbate at constant temperature is called Adsorption Isotherm.
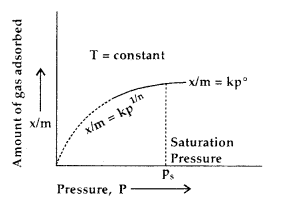
At low pressure \(\frac{x}{m}\) ∝ p1 ………..(i)
At high pressure \(\frac{x}{m}\) ∝ p0 ………..(ii)
In the intermediate range of pressure, combining (i) and (ii)
\(\frac{x}{m}\) ∝ p0-1
∝ p1/n where n is an integer (n > 1)
or
\(\frac{x}{m}\) = kp1/n ………..(iii)
where k is a constant depending upon the nature of the adsorbate and adsorbent.
This relationship is called Freundlich Adsorption Isotherm
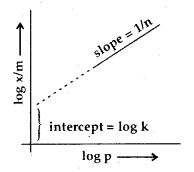
Taking logs on both sides of (iii)
log \(\frac{x}{m}\) = log k + \(\frac{1}{n}\) log p
A graph between log \(\frac{x}{m}\) against log p should, therefore, be a straight line with slope equal to \(\frac{1}{n}\) and ordinate intercept equal to log K.
Adsorption From Solution Phase:
Solids can adsorb solutes from solutions so when a solution of acetic acid in water is shaken with charcoal, a part of the acid is adsorbed by charcoal and the concentration of the acid decreases in the solution.
The following conclusions have been made regarding adsorption from solution phase:
- The extent of adsorption decreases with increase in temperature.
- The extent of adsorption increases with the increase in the surface area of the absorbent.
- The extent of adsorption depends upon the concentration of the solute in the solution.
- The extent of adsorption depends upon the nature of the absorbent and the adsorbate.
Freundlich equation as applied to solutions is modified as \(\frac{x}{m}\) = k C1/n i.e., where C is the equilibrium concentration,
or
log \(\frac{x}{m}\) = log k + \(\frac{1}{n}\) log C
Applications of Adsorption:
1. Production of high vacuum: The remaining traces of air can be adsorbed by charcoal from a vessel evacuated by a vacuum pump to give a very high vacuum.
2. Gas masks: Gas mask (a device which consists of activated charcoal or mixture of adsorbents) is usually used for breathing in coal mines to adsorb poisonous gases.
3. Control of humidity: Silica and aluminium gels are used as adsorbents for removing moisture and controlling humidity.
4. Removal of colouring matter from solutions: Animal charcoal removes colours of solutions by adsorbing coloured impurities.
5. Heterogeneous catalysis: Adsorption of reactants on the solid surface of the catalysts increases the rate of reaction. There are many gaseous reactions of industrial importance involving solid catalysts. Manufacture of ammonia using iron as a catalyst, manufacture of H2SO4 by contact process and use of finely divided nickel in the hydrogenation of oils are excellent examples of heterogeneous catalysis.
6. Separation of inert gases: Due to the difference in degree of adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
7. In curing diseases: A number of drugs are used to kill germs by getting adsorbed on them.
8. Froth floatation process: A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method using pine oil and frothing agent (see Unit 6).
9. Adsorption indicators: Surfaces of certain precipitates such as silver halides have the property of adsorbing some dyes like eosfn, fluorescein, etc. and thereby producing a characteristic colour at the end point.
10. Chromatographic analysis: Chromatographic analysis based on the phenomenon of adsorption finds a number of applications in analytical and industrial fields.
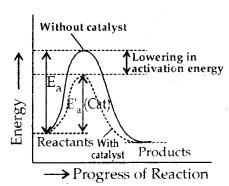
Catalysis: Potassium chlorate when heated strongly decomposes slowly giving dioxygen. The decomposition between 653 – 873 K.
2 KClO3 → 2 KCl + 3O2
However with a little of MnO a decomposition occurs only at 473 – 633 K and also at a much accelerated rate. MnO2 is a catalyst for this reaction.
![]()
A substance which accelerates the rate of a chemical reaction, itself remaining chemically and quantitatively unchanged after the reaction is called a catalyst.
Promoter is a substance that enhances the activity of a catalyst, while Poison is a substance that decreases the activity of a catalyst. In the reaction for the manufacture of NH3 by Haber’s process.
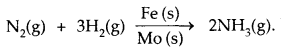
Fe is catalyst and Molybdenum (MO) is a promoter.
Homogeneous And Heterogeneous Catalysis:
When the reactants and the catalyst are in the same phase (liquid or gas), the process is said to be homogeneous catalysis.
Example:
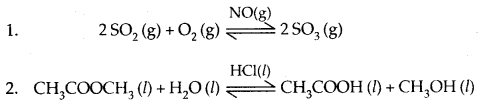
When the reactants and the catalyst are in different phases, the process is called heterogeneous catalysis.
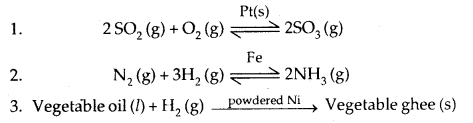
Important Features of solid catalysts are:
(a) Activity: It depends upon the strength of chemisorption to a large extent. .
(b) Selectivity: Selectivity of a catalyst is its ability to direct a reaction to yield a particular product. For example, starting with H2 and CO and using different catalysts, we get different products.
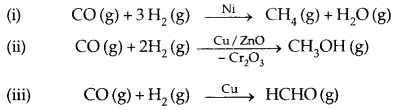
Thus a catalyst is highly selective in nature, i.e., a given substance can act as a catalyst only in a particular reaction and not for all the reactions. A catalyst for a particular reaction may fail to catalyse any other reaction.
Shape-Selective Catalysis by Zeolites: The catalytical reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape selective catalysis. Zeolites are good shape-selective catalysts because of their honey comb¬like structure.
An important Zeolite catalyst used in the petroleum industry is ZSM-5. It converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Enzyme Catalysis: Enzymes are complex nitrogeneous organic compounds which are produced by living plants and animals. They are actually protein molecules of high molecular mass, and form colloidal solutions in water. The enzymes are also referred to as Bio-Chemical Catalysts as they also occur in the bodies of animals and plants and such a phenomenon is known as Bio-Chemical Catalysis.
The following are examples of enzyme-catalysed reactions:
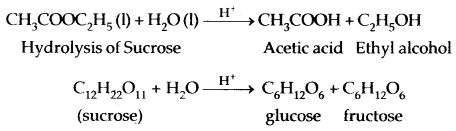
1. Inversion of cane-sugar
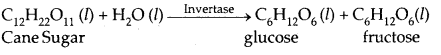
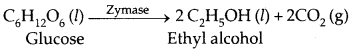
2. Conversion of glucose into ethyl alcohol.
3. Decomposition of urea into ammonia and CO2.
![]()
| Enzyme | Source | Enzymatic Reaction |
| Invertase | Yeast | Sucrose → Glucose and fructose |
| Zymase | Yeast | Glucose → Ethyl alcohol and carbondioxide |
| Diastase | Malt | Starch → Maltose |
| Maltase | Yeast | Maltose → Glucose |
| Urease | Soyabean | Urea → Ammonia and carbon dioxide |
| Pepsin | Stomach | Proteins → Amino acids |
Characteristics Of Enzyme Catalysis:
Enzyme catalysis is unique in its efficiency and high degree of specificity. The following characteristics are exhibited by enzyme catalysts:
1. Most highly efficient: One molecule of an enzyme may transform one million molecules of the reactant per minute.
2. Highly specific nature: Each enzyme is specific for a given reaction, i.e., one catalyst cannot catalyse more than one reaction. For example, the enzyme urease catalyses the hydrolysis of urea only. It does not catalyse hydrolysis of any other amide.
3. Highly active under optimum pH: The rate of an enzyme- catalysed reaction is maximum at a particular pH called optimum pH, which is between pH value 5-7.
4. Highly active under optimum temperature: The rate of an enzyme reaction becomes,maximum at a definite temperature, called the optimum temperature. On either side of the optimum temperature, the enzyme activity decreases. The optimum temperature range for enzymatic activity is 298-310 K. Human body temperature being 310 K is suited to enzyme-catalysed reaction.
5. Increasing activity in presence of activators and co¬enzymes: The enzymatic activity is increased in the presence of certain substances, known as co-enzymes. It has been observed that when a small non-protein (vitamin) is present along with an enzyme, the catalytic activity is enhanced considerably.
Activators are generally metal ions such as Na+ Mn2+, CO2+, Cu2+, etc. These metal ions, when weakly bonded to enzyme molecules, increase their catalytic activity. Amylase in presence of sodium chloride i.e., Na+ ions are catalytically very active.
6. Influence of inhibitors and poisons: Like ordinary catalysts enzymes are also inhibited or poisoned by the presence of certain substances. The inhibitors or poisons interact with the active functional groups on the enzyme surface and often reduce or completely destroy the catalytic activity of the enzymes. The use of many drugs is related to their action as enzyme inhibitors in the body.
Mechanism of Enzyme Catalysis: There are a number of cavities present on the surface of collodial particles of enzymes. These cavities are of characteristic shape and possess active groups such as – NH2, – COOH, – SH, – OH etc. These are actually the active centres on the surface of enzyme particles. The molecules of the reactant (substrate), which have complementary shape, fit into these cavities just like a key fits into a lock.
An activated complex is formed which then decomposes to yield the products in two steps as outlined below:
Step I: E + S → ES*
Step II: ES* → E + P
Some Industrial Catalytical Processes:
| Process | Catalyst |
| 1. Haber’s process for the manu-facture of ammonia N2(g) + 3H2(g) → 2NH3(g) | 1. Finely divided iron, molybdenum as promoter; conditions: 200 bar pressure and 723 – 773K temperature. |
| 2. Ostwald’s process for the manu-facture of nitric acid. 4NH3(g) + 5O2(g) → 4N0(g) + 6H2O(g) 2NO(g) + O2(g) → 2NO2(g) 4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(l) |
2. Platinised asbestos; temperature: 573K. |
| 3. Contact process for the manu-facture of sulphuric acid. 2SO2(g) + O2 (g) ⇌ 2SO3(g) SO3(g) + H2SO4 (l) → H2S2O7 (l)oleum H2S2O7 (l)+H2O (l) → 2H2SO4 (l) |
3. Platinised asbestos or vanadium pentoxide (V205); temperature 673-723K. |
Colloids: A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium.
The essential difference between a solution and a colloid is that of particle size. Their size is in between that of true solution and suspension
Classification of Colloids: Colloids are classified on the basis of the following criteria:
(a) Physical state of dispersed phase and dispersion medium.
(b) Nature of interaction between dispersed phase and dispersion medium.
(c) Type of particles of the dispersed phase.
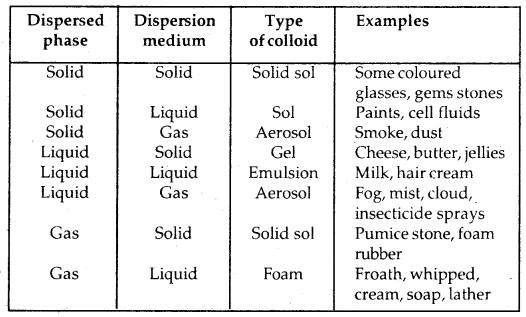
(a) Physical state of dispersed phase and dispersion medium: Depending upon whether the dispersed phase and the dispersion medium are solids, liquids or gases, eight types of colloidal systems are possible.
Types of Collodial Systems:
(b) Depending upon the nature of interactions between the dispersed phase and dispersion medium, the colloids can be classified as Lyophilic Colloids and Lyophobic Colloids.
1. Lyophilic collids: The colloidal solutions in which the particles of the dispersed phase have a great affinity (or love) for the dispersion medium are called lyophilic colloids. Such solutions are reversible in nature. In case water acts as the dispersion medium, the lyophilic colloid is called hydrophilic colloid. The common examples of lyophilic colloids are glue, gelatin, starch, proteins, rubber etc.
2. Lyophobic colloids: The colloidal solutions in which the particles of the dispersed phase have no affinity or love, rather have hatred for the dispersion medium, are called lyophobic collids. The solutions of metals like Ag and Au, hydroxides like Al (OH)3 and Fe (OH)3 and metal sulphides like As2S3 are examples of lyophobic colloids.
Such sols are formed with difficulty. They are irreversible in nature.
Multimolecular Macromolecular And Associated Colloids:
Depending upon the molecular size, the colloids can be classified as:
1. Multimolecular colloids: In this type, the particles consist of an aggregate of atoms or small molecules with molecular size less than 1 nm. For example, sols of gold atoms and sulphur (S8) molecules. In these colloids, the particles are held together by Van der Waals forces.
2. Macromolecular colloids: In this type, the particles of the dispersed phase are sufficiently big in size (macro) to be of colloidal dimensions. In this case, a large number of small molecules are joined together through their primary valencies to form giant molecules.
These molecules are called macro molecules and each macromolecule may consist of hundreds or thousands of simple molecules. The solution of such moleucles are called macromolecular soluions. For example, colloidal solution of starch, cellulose, etc.
3. Associated colloids: These are the substances which behave as normal electrolytes at low concentration but behave, as colloidal particles at higher concentration. These associated particles are also called miscelles. For example, in aqueous solution, soap (sodium stearate) ionises as:
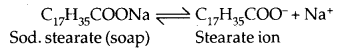
In concentrated solutions, these ions get associated to form an aggregate of colloidal size.
General Methods Of Preparation Of Sols:
Lyophilic sols are readily formed by simply mixing the dispersed phase and the dispersion medium under ordinary conditions.
Lyophobic sols can generally be prepared by two methods.
1. Condensation methods,
2. Dispersion methods.
→ Condensation methods: In these methods, the smaller particles are condensed suitably to be of colloidal size. This can be done by chemical reactions or by exchange of solvent.
→ Dispersion methods: In these methods, the large particles of a substance (suspension) are broken into smaller particles. This can be done by mechanical dispersion, by electrical dispersion or Bredig’s arc method and by peptisation.
Purification Of Colloidal Solutions:
The colloidal solutions prepared usually contain impurities especially electrolytes which can destabilize the sols. These impurities must be eliminated to make the colloidal solution stable.
The following methods are commonly used for the purification of colloidal solutions.
1. Dialysis: The method is based upon the fact that colloidal particles cannot pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it. The colloidal solution is taken in a bag made of cellophane or parchment.
The bag is suspended in fresh water. The impurities slowly diffuse out of the bag leaving behind pure colloidal solution. For example, dialysis can be used for removing HCl from the ferric hydroxide sol.
2. Electrodialysis: The ordinary process of dialysis is slow:
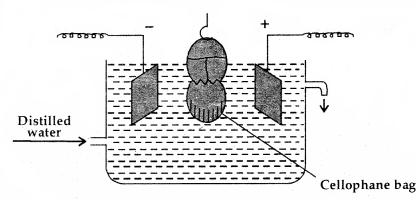
An apparatus for electrodialysis
To speed up the process of purification, the dialysis is carried out by applying electric field. This process is called electrodialysis.
3. Ultra-filtration: It is the process of removing the impurities from the colloidal solution by passing it through graded filter paper called ultrafilter papers. These filter papers are made from ordinary filter papers by impregnating them with colloidal solutions.
As a result, the size of the pores gets reduced. These filter papers allow the ions and molecules of the impurities to pass but retain colloidal particles. Ordinary filter papers cannot be used for this purpose since the colloidal particles also easily pass through the pores of these papers.
Properties Of Colloidal Solutions: The important properties of colloidal solutions are:
1. Heterogeneous nature: The colloidal solutions are heterogenous in nature consisting of dispersed phase and dispersion medium.
2. Visibility: The colloidals are not visible to naked eye and these can be seen with ultra microscopes.
3. Brownian movement: The colloidal particles have continuous zigzag motion called Brownian movement.
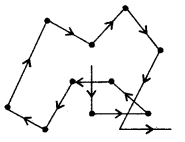
Brownian Movement
4. Tyndall effect: When the light is passed through the colloidal solution, the path of the light becomes visible when viewed from a direction at right angle to the incident beam. This phenomenon was studied by Tyndall and is known as Tyndall effect. The phenomenon of scattering oflight’by colloidal particles as a result of which the path of the beam becomes visible is called Tyndall effect.
5. Electrical properties: The particles of the colloidal solutions possess electrical charge, positive or negative. The presence of charge is responsible for the stability of these solutions. It may be noted that only the sol particles carry some charge while the dispersion medium has no charge. For example, the collodial solutions of gold, arsenious sulphide (AS2S3) are negatively charged while those of Fe (OH)3 and Al (OH)3 have positive charge. In the case of silver chloride sol, the particles may either be positively or negatively charged.
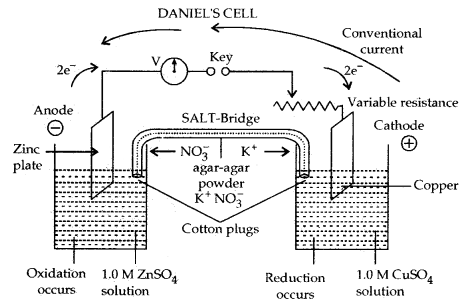
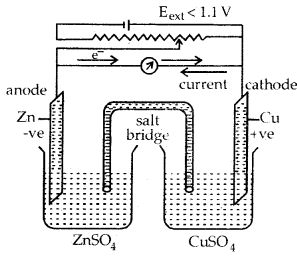
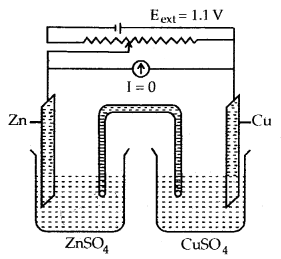
The presence of the charge on the sol particles and its nature whether positive of negative can be determined with the help of a phenomenon known as electrophoresis. In this experiment, the colloidal particles move towards positive or negative electrodes depending upon their charge under the influence of electrical field.
The phenomenon of movement of colloidal particles under an applied electric field is called electrophoresis.
If the particles accumulate near the negative electrode, the charge on the particles is positive. On the other hand, if the sol particles accumulate near the positive electrode the charge on the particles is negative.
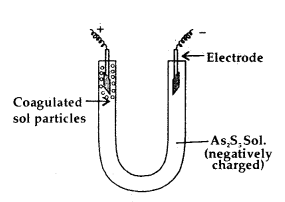
A set up for electrophoresis
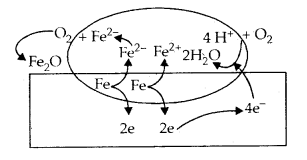
Origin of charge: The charge on the colloidal particles may be due to selective adsorption of ions. The particles contributing the dispersed phase adsorb only those ions preferentially which are common with their own lattice ions. For example, if silver nitrate solution is added to an aqueous solution of potassium iodide, the silver iodide will adsorb negative ions (I–) from the dispersion medium to form a negatively charged sol.
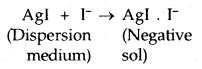
However, if silver iodide is formed by adding potassium iodide to silver nitrate solution, the sol will be positively charged due to the adsorption of Ag+ ions present in the dispersion medium.

Coagulation Of Colloidal Solution: The phenomenon of precipitation of a colloidal solution by the addition of excess of an electrolyte is called coagulation or floculation.
→ Factors governing coagulation
1. Nature of the electrolytes: The coagulation capacity of different electrolytes depends upon the valency cf the active ion or called floculating ion. It is the ion carrying charge opposite to the charge on the colloidal particles. According to Hardy Schulz law, greater the valency of the active ion or floculating ion greater will be its coagulating power. Thus, to coagulate negative sol of As2S3, the coagulating power of different cations has been found to decrease in the order as:
Al3+ > Mg2+ > Na+
Similarly, to coagulate a positive sol such as Fe (OH)3, the coagulating power of different anions has been found to decrease in the order:
[Fe(CN)6]4- > PO4 3- > SO42- > Cl–
The minimum concentration of an electrolyte which is required to cause the coagulation or flocculation of a sol is known as flocculation value. It is usually expressed as milli moles per litre.
Protection of Colloids: The process of protecting the lyophobic colloidal solution from precipitation by the electrolytes due to the previous addition of some lyophilic colloid is called protection. The colloid which is added to achieve such a protection is called protecting colloid.
Gold Number: The different protecting colloids differ in their portecting powers. Zsigmondy introduced a term called gold number to describe the protective power of different colloids. This is defined as the minimum number of milligrams of the protective colloid required to just prevent the coagulation of a 10 ml of a given gold sol when 1 ml of a 10% solution of sodium chloride is added to it.
The coagulation of gold sol is indicated by change incolour from red to blue. The gold number of a few protective colloids are as follows:

It may be noted that smaller the value of gold number, greater will be protecting power of the protective colloid. Therefore, reciprocal of gold number is a measure of the protective power of a colloid. Thus, out of the list given above, gelatin is the best protective colloid.
Emulsions: Emulsions are the colloidal solutions of two immiscible liquids in which the liquid acts as the dispersed phase as well as the dispersion medium. Normally they are obtained by mixing an oil with water. Since the two do not mix well, the emulsion is generally unstable and is stabilised by adding a suitable outside reagent called emulsifier or emulsifying agent. The substances that are commonly employed for the purposes are gum, soap, glass powder, etc.
Types of Emulsions: These are of two types:
1. Oil-in-water emulsions: In this case, oils acts as the dispersed phase (small amount) and water as the dispersion medium (excess) e.g., milk is an emulsion of soluble fats in water and here casein acts as an emulsifier. Vanishing cream is another example of this class. Such emulsions are called aqueous emulsions.
2. Water-in-oil emulsions: In this case water acts as the dispersed phase while the oil behaves as the dispersion medium e.g., butter, cod liver oil, cold cream etc. Such types of emulsions are called oily emulsions.
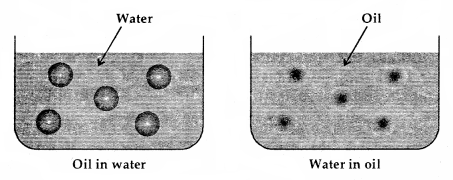
Types of Emulsions
Demulsification: It is the process of decomposing an emulsion back into its constituent liquids. The demulsification can be done by centrifugation, filtration, boiling, freezing and some chemical methods.
Following are the interesting noteworthy examples of colloids which we come across in daily life.
- Blue colour of the sky: Dust particles along with water suspended scatter blue light due to which sky looks blue to, us.
- Fog, mist and rain: Clouds are aerosols. It is possible to cause artificial rain by throwing electrified sand.
- Food articles like milk, butter, fruit juices are all colloids.
- Blood-: Blood is a colloidal solution of an albuminoid substahce. Alum and FeCl3 solution stop oozing blood due to coagulation. ,
- Soils: Fertile soils are colloidal in nature in which humus . acts as a protective colloid.
- Formation of delta: River water is a colloidal solution of clay.
Sea water contains several electrolytes. When river water meets sea water, the electrolytes present in sea water, coagulate the colloidal solution of clay resulting in its deposition with the formation of delta.
Applications of colloids: Colloids are widely used in the industry.
Some examples are:
1. Cottrell Smoke Precipitator: Smoke is a colloidal solution of solid particles such as carbon, arsenic compounds, dust etc. in air. The smoke is led through a chamber containing platgs having a charge opposite to that carried by smoke particles.” The particles on coming in contact with these plates lose their charge and get precipitated. The particles thus settle down on the floor of the chamber.
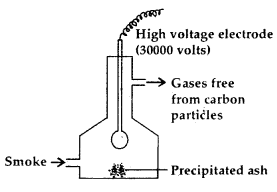
Cottrell Smoke Precipitator
2. Purification of drinking water: Alum is added to water to coagulate the suspended impurities present in water from natural resources to make it fit for drinking purposes.
3. Medicines: Most of the medicines are colloidal in nature. . Milk of magnesia, an emulsion, is used for stomach disorder. Argyrol is a silver sol used as an eye lotion. Colloidal antimony is used in curing Kalazar. Colloidal gold is used for intramuscular injection. Colloidal medicines are more effective because of large surface area and hence are easily assimilated.
4. Tanning: Animal hides due to colloidal nature bear positive charge when it is soaked in tanning or chromium salts which Contain negatively charged colloidal particles, mutual coagulation takes place leading to hardening of leather. The process is called tanning.
5. Cleansing action of soaps and detergents: Soaps and detergents act as emulsifiers and remove greasy impurities and dust of clothes which is washed away.
6. Photographic plates & films are prepared by coating an emulsion of light sensitive AgBr in gelatin over glass plates or celluloid films.
7. Rubber industry: Latex is a colloidal solution of rubber particles which are negatively charged. Rubber is ob tained by coagulation of latex.
8. Industrial products: Paints, inks, synthetic plastics and rubber, graphite lubricants, cement etc. are all colloidal solutions.