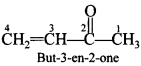
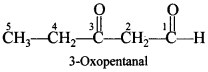
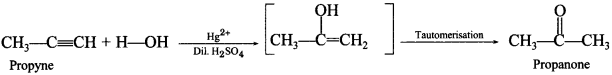
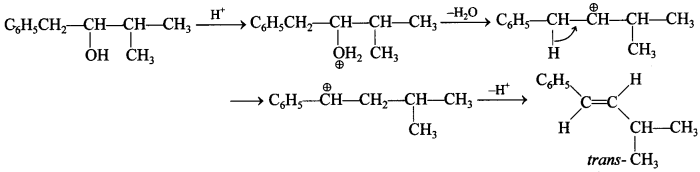
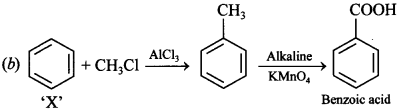
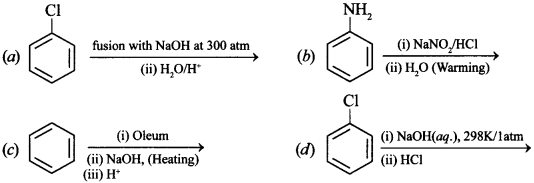
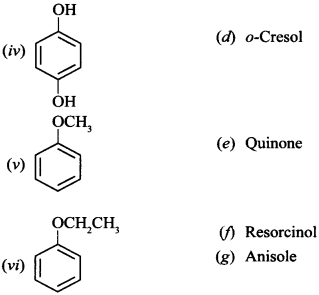
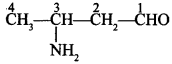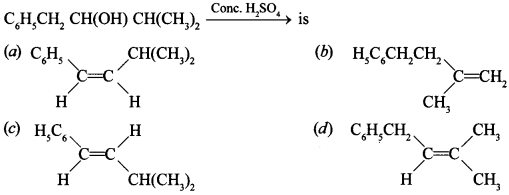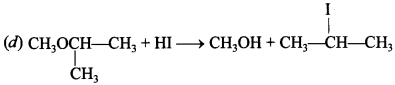Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 16 Chemistry in Everyday Life. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Chemistry in Everyday Life MCQs Pdf with Answers to know their preparation level.
Chemistry in Everyday Life Class 12 Chemistry MCQs Pdf
Chemistry MCQS for Class 12 Question 1. Which of the following is used as tranquilizer?
(a) Naproxene
(b) Tetracycline
(c) Chlorpheniramine
(d) Equanil
Answer/Explanation
Answer: d
Explaination: (d) Equanil.
2. Which one of the following is antihistamine?
(a) Chloramphenicol
(b) Diphenyl hydramine
(c) Norethindrone
(d) Omeprazole
Answer/Explanation
Answer:
Explaination:
(b) (a) is broad spectrum antibiotic
(c) is ’ antifertility drug (birth control) and
(d) is antacid.
3. Artificial sweetener which is stable only under cold conditions only is
(a) saccharine
(b) sucralose
(c) aspartame
(d) alitame
Answer/Explanation
Answer: c
Explaination: (c) aspartame.
Chemistry MCQS for Class 12 with Answers Pdf Question 4. Bithional added to soap acts as
(a) buffering agent
(b) antiseptic
(c) softener
(d) drying
Answer/Explanation
Answer: b
Explaination: (b) antiseptic.
5. .Which of the following is analgesic?
(a) Streptomycin
(b) Chloromycetin
(c) Novalgin
(d) Penicillin
Answer/Explanation
Answer: c
Explaination: (c) is analgesic (pain reliever) others are antibiotics.
6. Mixture of chloroxylenol and terpineol acts as
(a) antiseptic
(b) antipyretic
(c) antibiotic
(d) analgesics
Answer/Explanation
Answer: a
Explaination: (a) These are components of Dettol.
Class 12 Chemistry MCQ Pdf Question 7. Which of the following is used as antacid?
(a) Iproniazid
(b) Salvarsan
(c) Zantac
(d) Chloramphenicol
Answer/Explanation
Answer:
Explaination:
(c) Zantac is antacid,
(b) is used for treatment of syphilis,
(a) is tranquilizer and
(d) is broad spectrum antibiotic.
8. Drugs that bind to receptor site and inhibit its natural function are called
(a) antagonists
(b) agonists
(c) enzymes
(d) molecular targets
Answer/Explanation
Answer: a
Explaination: (a) These are called antagonists.
9. The drug Tagamet is
(a) analgesics
(b) antidepressant
(c) anaesthetic
(d) antacid
Answer/Explanation
Answer: d
Explaination: (d) antacid.
10. Which of the following cationic detergent?
(a) Sodium lauryl sulphate.
(b) Cetyl trimethyl ammonium bromide.
(c) Sodium dodecyl benzene sulphonate.
(d) Glyceryl oleate.
Answer/Explanation
Answer:
Explaination:
(b) (a) and (c) are anionic detergents and
(d) is fat (glycerol ester of fatty acid).
11. The artificial sweetener which contains chlorine that has the appearance and taste as that of sugar and is stable and cooking temperature:
(a) Aspartame
(b) Saccharin
(c) Sucralose
(d) Alitame
Answer/Explanation
Answer: c
Explaination: (c) Sucralose
12. Narcotic analgesic is
(a) aspirin
(b) paracetamol
(c) codeine
(d) cimetidine
Answer/Explanation
Answer:
Explaination:
(c). (a) and (b) are analgesics and antipyretic
(d) is antacid.
13. Bactericidal antibiotic among the following is
(a) ofloxacin
(b) erythromycin
(c) chloramphenicol
(d) tetracycline
Answer/Explanation
Answer: a
Explaination:
(a) is bactericidal, others are bacteriostatic, i.e., suppress the multiplication of bacteria.
14. Butylated Hydroxy Toluene (BHT) as a food additive acts as
(a) antioxidant
(b) flavouring agent
(c) colouring agent
(d) emulsifier
Answer/Explanation
Answer: a
Explaination: (a) It prevents spoilage of butter.
15. Sodium benzoate is used as
(a) food preservative
(b) artificial sweetener
(c) antioxidant
(d) detergent
Answer/Explanation
Answer: a
Explaination: (a) It is food preservative, used in cold drinks.
16. Which of the following is not an antacid?
(a) Phenelzine
(b) Ranitidine
(c) Al(OH)3
(d) Cimetidine
Answer/Explanation
Answer: a
Explaination:
(a) Phenelzine is antidepressant (tranquilizer), not an antacid, others are antacids.
17. Allergy is caused by the production of in the body
(a) Hormones
(b) Enzymes
(c) Vitamins
(d) Histamines
Answer
Answer: d
18. Which of the following can possibly be used as analgesic without causing addiction and modification?
(a) Morphine
(b) Diazepam
(c) N-acety 1-para-aminophenol
(d) LSD
Answer
Answer: c
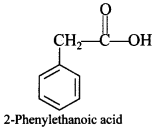
Chemistry MCQ with Answers Class 12 Question 19. Aspiring is an acetylation product of ______
(a) p-Dihydroxybenzene
(b) o-Hydroxybenzoic acid
(c) o-Dihydroxy benzene
(d) m-Hydroxy benzoic acid
Answer
Answer: b
20. Drug which is used to reduce anxiety and brings calmness is known as
(a) Tranquilizer
(b) Diuretic
(c) Analgesic
(d) Antacids
Answer
Answer: a
21. Which element is not present in Saccharin, an artificial sweetner?
(a) C
(b) P
(c) S
(d) N
Answer
Answer: b
22. Heroin is
(a) Narcotic
(b) Non-narcotic
(c) Anaesthetic
(d) Antiseptic
Answer
Answer: a
23. Detergents are better than soaps because
(a) They are less affected by hard water
(b) They can be used in acidic solution
(c) Both (a) and (b)
(d) They wash clothes better
Answer
Answer: c
24. Which one is a broad spectrum drug?
(a) Chloramphenicol
(b) Chloroquine
(c) Chloroxylenol
(d) Plasmoquine
Answer
Answer: a
25. A drug that is antiseptic as well as analgesic is
(a) Para acetamidophenol
(b) Chloropromazine hydrochloride
(c) Chloramphenicol
(d) Paracetamol
Answer
Answer: a
Chemistry MCQS for Class 12 Chapter wise Pdf Question 26. Match List-I with List-II and select the correct answer using the code given below in the Lists.

(a) 1 -(a), 2-(c), 3-(d), 4-(e)
(b) 1 -(b), 2-(a), 3-(d), 4-(e)
(c) 1 -(b), 2-(c), 3-(e), 4-(d)
(d) 1 -(b), 2-(a), 3-(d), 4-(e)
Answer
Answer: d
27. A narrow spectrum antibiotic is active against __________ .[NCERT Exemplar]
(a) gram positive or gram negative bacteria.
(b) gram negative bacteria only.
(c) single organism or one disease.
(d) both gram positive and gram negative bacteria.
Answer/Explanation
Answer: a
Explaination:
(a) is correct statement.
28. Which of the following enhances leathering property of soap? [NCERT Exemplar]
(a) Sodium carbonate
(b) Sodium rosinate
(c) Sodium stearate
(d) Trisodium phosphate
Answer/Explanation
Answer: b
Explaination:
(b) Sodium rosinate enhance lathering property of soap.
29. Glycerol is added to soap. It functions __________ . [NCERT Exemplar]
(a) as a filler.
(b) to increase leathering.
(c) to prevent rapid drying.
(d) to make soap granules.
Answer/Explanation
Answer: c
Explaination:
(c) It prevents rapid drying because it is hygroscopic (absorbs moisture) from atmosphere.
30. Which of the following is an example of liquid dish-washing detergent? [NCERT Exemplar]

Answer/Explanation
Answer:
Explaination:
(b) is dishwashing, non-ionic detergent,
(d) Cationic,
(a) and (c) are anionic detergents.
31. Which of the following is not a target molecule for drug function in body? [NCERT Exemplar]
(a) Carbohydrates
(b) Lipids
(c) Vitamins
(d) Proteins
Answer/Explanation
Answer: c
Explaination:
(c) Vitamins are not a target molecules for drug function in body.
Note: In the following questions two or more options may be correct. (Q.22 to Q.25)
32. Which of the following statements are incorrect about receptor proteins? [NCERT Exemplar]
(а) Majority of receptor proteins are embedded in the cell membranes.
(b) The active site of receptor proteins opens on the inside region of the cell.
(c) Chemical messengers are received at the binding sites of receptor proteins.
(d) Shape of receptor doesn’t change during attachment of messenger.
Answer/Explanation
Answer:
Explaination:
(b) and (d) are incorrect.
33. Which of the following statements are correct about barbiturates? [NCERT Exemplar]
(a) Hypnotics or sleep producing agents.
(b) These are tranquilizers.
(c) Non-narcotic analgesics.
(d) Pain reducing without disturbing the nervous system.
Answer/Explanation
Answer:
Explaination:
(a) and (b) are correct.
34. Compounds with antiseptic properties are __________ . [NCERT Exemplar]
(a) CHCl3
(b) CHI3
(c) Boric acid
(d) 0.3 ppm aqueous solution of Cl2
Answer/Explanation
Answer:
Explaination:
(b) CHI3 (Iodoform) and
(c) Boric acid are antiseptics.
35. Which of the following statements are [NCERT Exemplar]
(a) An antibacterial fungus.
(b) Ampicillin is its synthetic modification.
(c) It has bacteriostatic effect. soap bubbles .
(d) It is a broad spectrum antibiotic.
Answer/Explanation
Answer:
Explaination:
(c) and (d) are incorrect. It is bactericidal.
36. Match the medicines given in Column I with their use given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Ranitidine | (i) Tranquilizer |
| (b) Furacine | (ii) Antibiotic |
| (c) Phenelzine | (iii) Antihistamine |
| (d) Chloramphenicol | (iv) Antiseptic |
| (v) Antifertility drug |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (iv)
(c) (i)
(d) (ii)
37. Match the soaps given in Column I with incorrect about penicillin? items given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Soap chips | (i) dried miniature |
| (b) Soap granules | (ii) small broken pieces of soap formed from melted soaps |
| (c) Soap powder | (iii) soap powder + abrasives + builders (Na2CO3, Na3PO4) |
| (d) Scorning soap | (iv) soap powder + builders like Na2CO3 and Na3PO4 |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (i)
(c) (iv)
(d) (iii)
38. Match the class of compounds given in Column I with their functions given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Antagonists | (i) Communicate message between two neurons and that between neurons to muscles |
| (b) Agonists | (ii) Bind to the receptor site and inhibit its natural function |
| (c) Chemical messenger | (iii) Crucial to body’s communication process |
| (d) Inhibitors | (iv) Mimic the natural messenger |
| (e) Receptors | (v) Inhibit activities of enzymes. |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (iv)
(c) (i)
(d) (v)
(e) (iii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.29 to Q.32)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
39. Assertion: Sulpha drug contain sulphonamide group. [NCERT Exemplar]
Reason: Salvarsan is a sulpha drug.
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement.
40. Assertion: Transparent soaps are made by dissolving soaps in ethanol. [NCERT Exemplar]
Reason: Ethanol makes things invisible.
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement.
41. Assertion: Competitive inhibitors compete with natural substrate for their attachment on the active sites of enzymes. [NCERT Exemplar]
Reason: In competitive inhibition, inhibitor binds to the allosteric site of the enzyme.
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement.
42. Assertion: Non-competitive inhibitor inhibits the catalyic activity of enzyme by binding with its active site. [NCERT Exemplar]
Reason: Non-competitive inhibitor changes the shape of the active site in such a way that substrate can’t recognise it.
Answer/Explanation
Answer: d
Explaination:
(d) Assertion is wrong but reason is correct statement.
43. Histamine is potent __________ .
Answer/Explanation
Answer:
Explaination: vasodilator.
44. __________ prevents heart attack, can also be used as analgesics and antipyretic but should not be taken empty stomach as it releases salicylic acid, may damage stomach lining.
Answer/Explanation
Answer:
Explaination: Aspirin.
45. Plenty of salt and cover of oil act as __________ .
Answer/Explanation
Answer:
Explaination: preservative.
46. Bathing soaps are __________ salts of long chain fatty acids.
Answer/Explanation
Answer:
Explaination: potassium.
47. Aspirin is not suitable for ulcer patients. [True\False]
Answer/Explanation
Answer:
Explaination:
True, it forms salicylic acid which may cause bleeding and wounds.
48. cis-platin is used in chemotherapy for cancer. [True\False]
Answer/Explanation
Answer:
Explaination:
True, it is antitumour agent.
49. A person suffering from depression has low level of noradrenaline. [True\False]
Answer/Explanation
Answer:
Explaination: True.
50. Morphine is narcotic analgesics given in acute pain of terminal cancer. [True\False]
Answer/Explanation
Answer:
Explaination:
True, it causes addiction, should not be used regularly
51. Which site of an enzyme is called allosteric site?
Answer/Explanation
Answer:
Explaination:
Sites different from active site of enzyme ‘ where a molecule can bind and affect the active site is called allosteric site. Some drugs may also bind at this site.
52. Where are receptors located?
Answer/Explanation
Answer:
Explaination:
Receptors are embedded in cell membrane.
53. What is the harmful effect of hyperacidity?
Answer/Explanation
Answer:
Explaination:
It causes pain and may lead to formation of ulcer in stomach.
54. Write the name of an antacid which is often used as a medicine.
Answer/Explanation
Answer:
Explaination:
Ranitidine or NaHCO3 or a mixture of Al(OH)3 and Mg(OH)2 is used as antacid.
55. Give an example of antihistamine.
Answer/Explanation
Answer:
Explaination:
Brompheniramine (Dimetapp).
56. Which class of drugs is used in sleeping pills? [Delhi 2013]
Answer/Explanation
Answer:
Explaination:
Tranquilizers, (hypnotics)
57. Define the following and give one example: Tranquilizers [Delhi 2017,12]
Answer/Explanation
Answer:
Explaination:
Tranquilizers: Those drugs which reduce anxiety and produce a feeling of well being, e.g., Equanil, seconal, etc.
58. What is the cause of a feeling of depression in human beings? Name a drug which can be useful in treating this depression. [AI2012]
Answer/Explanation
Answer:
Explaination:
If the level of noradrenaline is low, then signal sending activity is low. It leads to depression. These drugs inhibit enzymes which catalyse
the degradation of noradrenaline which is neurotransmitter and thus, counteract the effect of depression. Chlordiazepoxide and meprobamate are mild tranquilizers suitable for relieving tension. Equanil is used in controlling depression and hypertension.
59. What are the uses of narcotic analgesics?
Answer/Explanation
Answer:
Explaination:
These drugs are mainly used for the relief of cardiac pain, pains of terminal cancer, child birth, etc.
60. Which type of drugs come under antimicrobial drugs?
Answer/Explanation
Answer:
Explaination:
Antiseptics, antibiotics and disinfectants are antimicrobial drugs.
61. What is the commonality between the antibioitic arsphenamine and azodye?
Answer/Explanation
Answer:
Explaination:
Arsphenamine possesses —As=As— linkage that resembles —N=N— linkages in azodye.
62. What is meant by a ‘broad spectrum antibiotic’? [Delhi 2019,17,14(C)]
Answer/Explanation
Answer:
Explaination:
Those antibiotics which are effective for large number of microorganisms are called ‘broad spectrum antibiotics’, e.g. chloramphenicol, tetracycline, etc.
63. What is meant by ‘narrow spectrum antibiotics’? Give one example. [Delhi 2017,13(C); Foreign 2012]
Answer/Explanation
Answer:
Explaination:
Those antibiotics which are effective against only particular micro-organism are called narrow spectrum antibiotics, e.g. Ampicillin, Streptomycin, etc.
64. Describe the following types of substances, giving suitable examples: Antiseptics [Chennai, Uttarakhand 2019; Delhi 2017]
Answer/Explanation
Answer:
Explaination:
Antiseptics: Those chemicals which prevent the growth of micro-organisms are called antiseptics, e.g. Dettol, 0.2% solution of phenol.
65. Give an example of disinfectant.
Answer/Explanation
Answer:
Explaination:
SO2 in very low concentration, 0.1% phenol, KMnO4
66. Give an example of antifertility drug.
Answer/Explanation
Answer:
Explaination: Novestrol.
67. State a reason of the following statement: The use of the sweetner aspartame is limited to cold foods and drinks. [All India 2014(C)]
Answer/Explanation
Answer:
Explaination: Because it is unstable at high temperature.
68. Name a food preservative which is most commonly used by food producers.
Answer/Explanation
Answer:
Explaination: Common salt is used as food preservative.
69. Give an example of antioxidant.
Answer/Explanation
Answer:
Explaination: Butylated hydroxy toluene (BHT).
70. Why soaps are biodegradable whereas detergents are non-biodegradable? [Delhi 2019]
Answer/Explanation
Answer:
Explaination:
Soaps are 100% biodegradable because it is decomposed by micro-organisms and does not create water pollution.
71. What is a soft soap?
Answer/Explanation
Answer:
Explaination:
Soft soaps are potassium salts of fatty acids.
72. Describe and illustrate with an example, a detergent.
Answer/Explanation
Answer:
Explaination:
Detergent is sodium or potassium salt of benzene sulphonic acid or sulphonate of unsaturated hydrocarbons of alkene type, e.g., sodium alkylbenzene sulphonate.
73. Which category of the synthetic detergents is used in toothpaste? [Delhi 2019]
Answer/Explanation
Answer:
Explaination: Anionic detergents.
74. Hair shampoos belong to which class of synthetic detergent?
Answer/Explanation
Answer:
Explaination: Cationic detergents.
75. Dishwashing soaps are synthetic detergents. What is their chemical nature?
Answer/Explanation
Answer:
Explaination: Non-ionic detergents.
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 16 Chemistry in Everyday Life will help you. If you have any query regarding CBSE Class 12 Chemistry Chemistry in Everyday Life MCQs Pdf, drop a comment below and we will get back to you at the earliest.




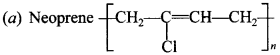





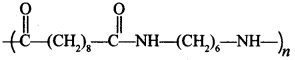






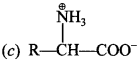







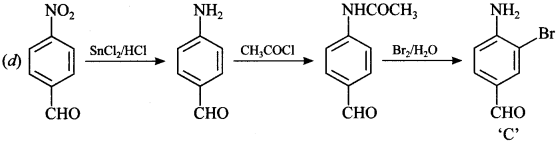
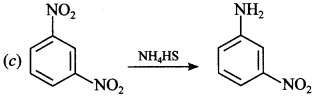









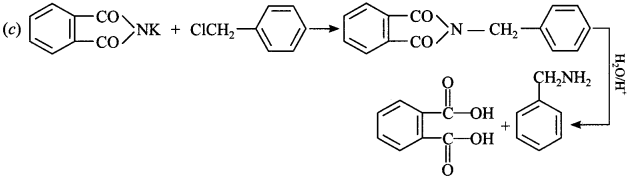







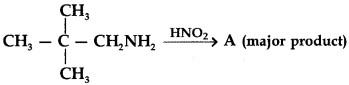
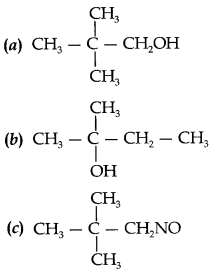

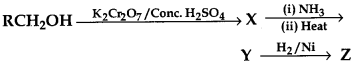



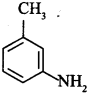



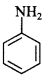 C6H5 group is electron withdrawing which decreases electron density on ‘N’.
C6H5 group is electron withdrawing which decreases electron density on ‘N’.
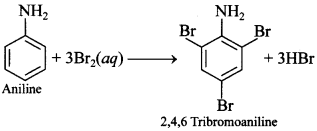









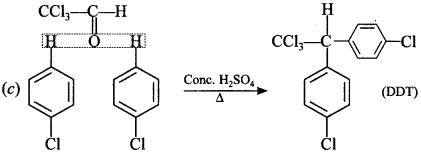

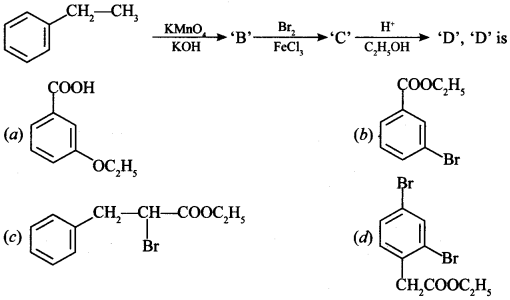

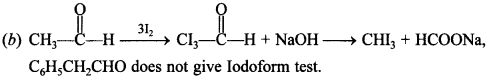



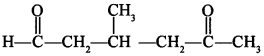

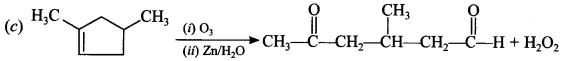









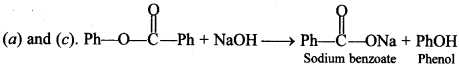



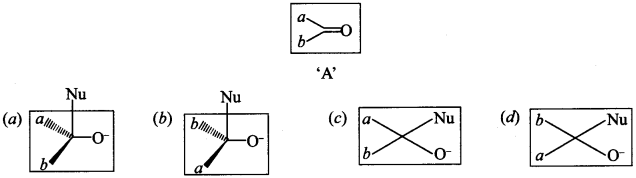

 [True/False]
[True/False]