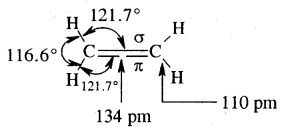
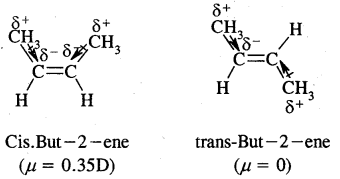
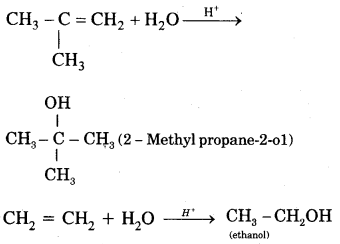
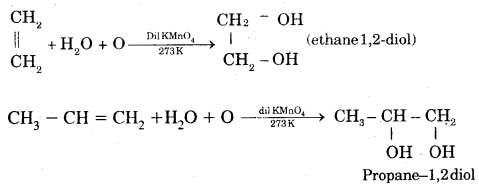
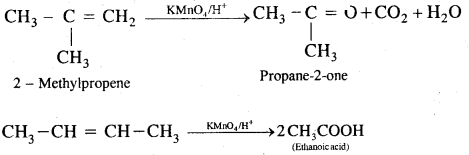
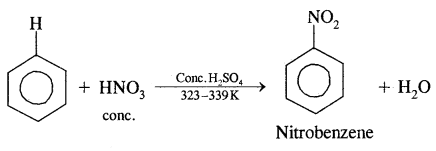
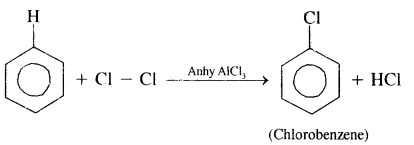
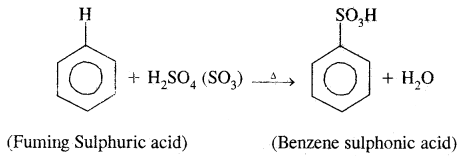
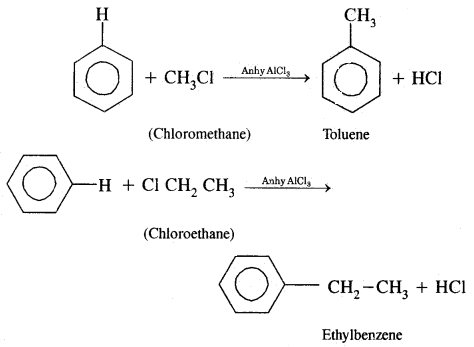
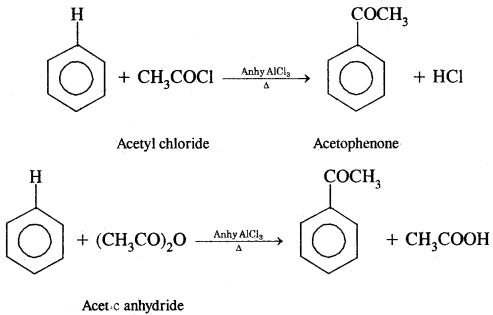
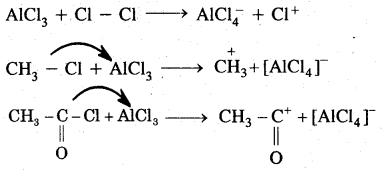
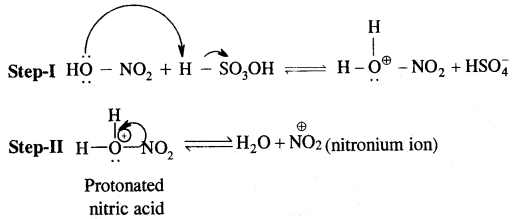
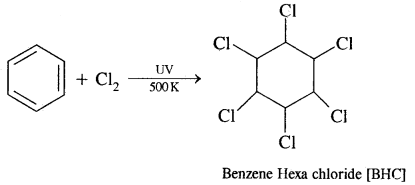
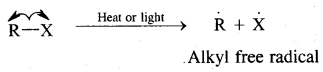
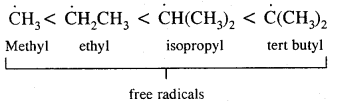
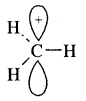
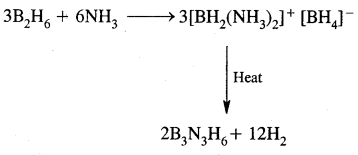
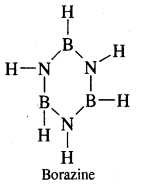
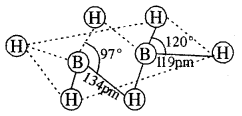
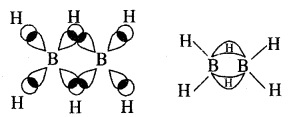
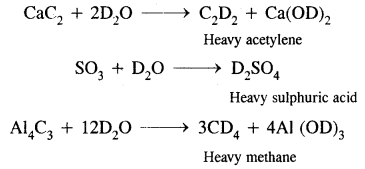
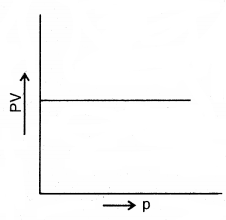
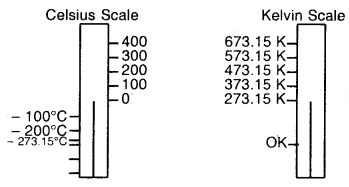
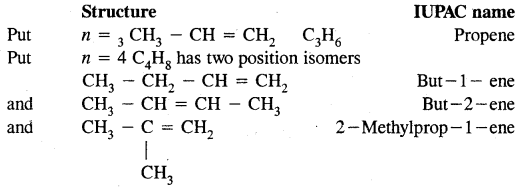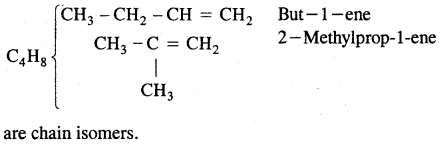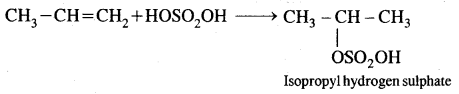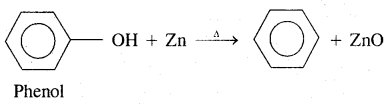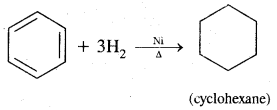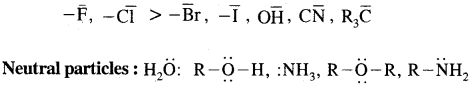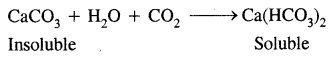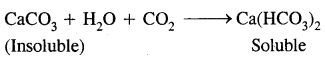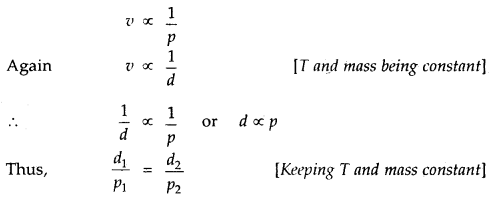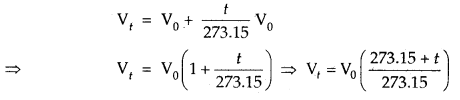By going through these CBSE Class 11 Chemistry Notes Chapter 14 Environmental Chemistry, students can recall all the concepts quickly.
Environmental Chemistry Notes Class 11 Chemistry Chapter 14
→ Environmental Pollution: Atmospheric, Tropospheric & stratospheric.
→ Water Pollution: Causes of water pollution & International standards of drinking water.
→ Soil Pollution: Pesticides-Intecticides & Herbecides & Fungicides
→ Industrial wastes: Recycling of wastes
→ Strategies to Control Environmental Pollution: Waste management.
→ Green Chemistry: Green Chemistry in day to day life.
→ Environmental Pollution: Environmental pollution is the effect of undesirable changes in the surrounding that have harmful effects on animals & plants & human beings.
→ Atmospheric pollution: Tropospheric & stratospheric.
→ Global warming: About 75 % of solar energy reaching the earth is absorbed by the earth’s surface, which increases its temperature. The rest of the heat radiates back to the atmosphere. Some of the heat is trapped by gases such as CO2, CH4 & O3 & C.F.Cs. & water vapours in the atmosphere. Thus they add to the heating of the atmosphere. This causes Global Warming.
→ Green House Effect: Atmosphere traps the sun’s heat near the earth’s surface & keeps it warm. This is called the Greenhouse effect because this makes the earth perfect for life.
→ Acid Rain: When the pH of rainwater drops below 5.6, it becomes acidic. Acid rain is caused by the presence of oxides of N & O in the atmosphere.
Acid rain is harmful to agriculture, trees & plants & washes away nutrients needed for their growth,
→ Classical Smog: It is a mixture of smoke, fog & SO2 chemically it is a reducing mixture & so it is called reducing smog.
→ Photochemical smog: The main components of the photochemical smog result from the action of sunlight on unsaturated hydrocarbons & nitrogen oxides produced by automobiles & factories. Photochemical smog has a high concentration of oxidizing agents & is, therefore, called oxidizing smog.
→ Ozone hole: In October 1979, A zone of lowered ozone concentration was detected over Antarctica which occurs mainly during September-October & gets replenished during November-December. This is called the ozone hole.
→ B.O.D.: Biochemical oxygen demand.
→ C.O.D.: Chemical oxygen demand.
→ Green Chemistry: It involves processes & products that reduce or eliminate the use or the generation of hazardous substances.
Chapter In Brief:
Environment Pollution:
It is defined as the effect of undesirable changes in our surroundings due to natural sources or human activity that have harmful effects on plants, animals and human beings. A.substance, which causes pollution to one or more components of the ecosystem are called pollutants. Pollutants can be solid, liquid, or gas.
Pollutants are of two types.
1. Non-Biodegradable Pollutants: The materials which do not undergo degradation or degrade very slowly in the environment like DDT, plastic materials, heavy metals, many chemicals, nuclear wastes etc.
2. Biodegradable Pollutants: The materials which are easily decomposed by the micro-organisms either by nature or by suitable treatment are called biodegradable pollutants. Examples; Domestic waste like discarded vegetables, cow dung etc.
→ Atmospheric Pollution: The lowest region of the atmosphere in which human beings along with other organisms live is called the troposphere. It extends up to the height of- 10 km from sea level. Above the troposphere between 10-15 km above sea level lies the stratosphere. Atmospheric pollution is generally studied as tropospheric and stratospheric pollution.
→ Tropospheric Pollution: It occurs due to the presence of undesirable solid or gaseous particles in the air.
1. Gaseous air pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone etc.
2. Particulate pollutants: These are dust, mist, fumes, smoke and smog”.
1. Gaseous air pollutants
(a) Oxides of sulphur are produced when sulphur-containing fossil fuel is burnt. SO2 is poisonous to both animals and plants. Particulate matter present in polluted air catalyses the oxidation of SO2 to SO3.
2SO2(g) + O2(g) → 2 SO3(g)
The reaction can also be promoted by ozone and hydrogen peroxide.
SO2(g) + O3(g) → SO3(g) + O2(g)
SO2(g) + H2O2 → H2SO4
(b) Oxides of Nitrogen: At high altitudes when lightning strike’s N2 and O2 present in the air combine to form nitric oxide.
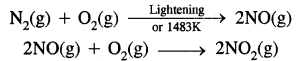
Rate of formation of NO2 Then NO reacts with O3 is faster.
NO(g) + O3(g) → NO(g) + O2(g)
The irritant red haze in the traffic and congested places is due to oxides of nitrogen. NO2 is a lung irritant.
(c) Hydrocarbons are formed by incomplete combustion of fuel used in automobiles. They are carcinogenic.
(d) Oxides of carbon
1. Carbon monoxide (CO) is one of the most serious air
pollutants. It is highly toxic. It blocks the delivery of oxygen to the organs and tissues. It is produced as a result of incomplete combustion of carbon
C(s) + \(\frac{1}{2}\)O2(g) → CO(g)
Insufficient quantity
It binds to haemoglobin for which it has 200-300 times more affinity than oxygen and forms carboxy-haemoglobin thus the oxygen-carrying capacity of blood is greatly reduced.
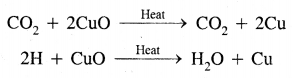
2. Carbon dioxide (CO2): It is released into the atmosphere by respiration and burning of fossil fuels. It is also emitted during volcanic eruptions and limestone burning to produce lime for cement plants. Normally a percentage of CO2 in the atmosphere is only 0.03 by volume. The increased amount of CO2 in the air is mainly responsible for global warming.
Global Warming and Greenhouse Effect:
Some of the heat from solar energy is trapped by gases such as carbon dioxide, methane (CH4), ozone, chlorofluorocarbons compounds (CFCs) and water vapours in the atmosphere. They add to the heating of the atmosphere. This causes global warming.
The natural greenhouse where there is an ecological balance of CO2, water vapours which are continuously being converted to carbohydrates and a fresh supply of oxygen during photosynthesis by a lot of green plants, flowers, vegetables makes the earth perfect for life. Sun’s warmth is retained on the earth giving sufficient heat to the soil and plants which emit infrared radiations.
If the amount of CO2 crosses the delicate 0.03% the natural greenhouse balance is disturbed. It is a major contributor to global warming. CFCs are damaging the ozone layer. Other contributors to global warming are CH4, N2O, and ozone. Cutting down forests and trees, burning fossil fuels, production of fertilizers, man-made industrial chemicals etc. add to global warming.
Acid Rain:
Due to the presence of CO2 in the atmosphere, the pH of rainwater is 5.6.
H2O(l) + CO2(g) ⇌ H2CO3(aq)
H2CO3(aq) ⇌ H+(aq) + HCO3 (aq)
When the pH of rainwater falls below 5.6, it is called Acid Rain Various human activities release oxides of Nitrogen and Sulphur in the atmosphere. SO2 and NO2 are thus major contributors to acid rain.
2SO2(g) + O2(g) + 2H2O(l) → 2H2SO4 (aq)
4NO2(g) + O2(g) + 2H2O(g) → 4HNO3(aq)
Acid rain is harmful:
- For plants, trees and agriculture as it washes away nutrients needed for their growth.
- For human beings and animals as it causes respiratory ailments.
- An aquatic ecosystem is disturbed when this add rain reaches water objects like rivers, lakes etc.
- It corrodes water pipes resulting in the leaching of heavy metals such as iron, lead and copper into drinking water.
- Acid rain damages buildings and other structures made of stone or metal. The Taj Mahal is being corroded by acid rain.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
As a result, the beautiful white marble of which the Taj Mahal was built centuries ago is rendered lusterless and is being slowly eaten away.
Particulate Pollutants:
They are of two types.
1. The Viable Particulates: They are bacteria, fungi, moulds, algae etc. which are minute living organisms that are dispersed in the atmosphere. They cause allergy to human beings and bring disease to plants.
2. Non-Viable Particulates: They are classified according to their nature and size as follows:
(a) Smoke particulates are solid or a mixture of solid and liquid particles formed during combustion of organic matter,
(b) Dust particles. Over 1 mm in diameter produced during the crushing, grinding and attribution of solid materials, sawdust, sand and cement particles, fly ash, dust storm etc. are the typical examples of this type of particulate emission.
(c) Mists are produced by particles of spray liquids and by condensation of vapours in air, Examples are H2S04 mist, herbicides and insecticides.
(d) Fumes coming out from chemical plants, sublimation, distillation, generally organic solvents, metals and metal oxides form fume particles.
The effect of particulate pollutants are largely dependent on particle size, They are all dangerous to human health.
Smogs:
The word smog is derived from smoke and fog. This is the major cause of air pollution.
Smogs are of two types:
(a) Classical Smog
(b) Photochemical Smog
(a) Classical Smog: It occurs in a cool humid climate. It is a mixture of smoke, fog, and SO2. Because of its reducing nature, it is also called Reducing Smog.
(b) Photochemical Smog: It occurs in a warm, dry and sunny climate. The main components result from the action of sunlight on unsaturated hydrocarbons and oxides of nitrogen. Since it has a high concentration of oxidizing agents, it is called Oxidizing Smog.
Formation of Photo Chemical Smog:
When both unsaturated hydrocarbons (unburnt fuels) and nitric oxide (NO) build-up to sufficiently high levels, a chain reaction occurs in the presence of sunlight.
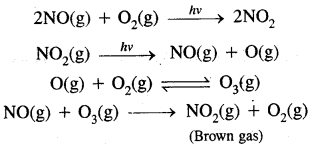
Ozone is a toxic gas. Both NO2 and O3 are oxidising agents.
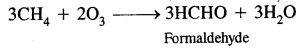
They further produce chemicals like acrolein (CH2 = CH CHO) and peroxyacetyl nitrate (CH3 CO3 NO2) or PAN
→ Effects of Photochemical Smog: Photochemical smog causes serious health problems. Both O3 and PAN present in photochemical smog cause extreme irritation to the eyes. O3 and NO irritate nose and throat and their high concentration causes a headache, chest pain, dryness of throat, cough and difficulty in breathing. It causes extensive damage to plant life, causes corrosion of metals, building materials, cracking of rubber etc.
→ Control: Photochemical smog can be controlled by controlling—the production of oxides, of nitrogen, controlling the burning of fossil fuels, using catalytic converters in automobiles etc. Certain plants also metabolise NO and therefore their plantation can help.
Stratospheric Pollution
Formation and Breakdown of Ozone: A large amount of ozone (O2) is present in the stratosphere. O3 protects us from the harmful UV rays coming from the sun.
→ Reactions occurring in the stratosphere: The main reactions occurring in the stratosphere. In this region ozone is formed in two steps: In the first step ultraviolet radiation coming from the sun have sufficient: energy to split dioxygen into two oxygen atoms. In the second step, the oxygen atoms react with more dioxygen to form ozone.
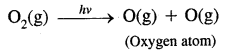
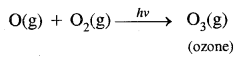
This ozone absorbs ultraviolet radiation and breaks down into dioxygen and an oxygen atom. Heat is given off, which warms up the stratosphere. Thus there is a dynamic equilibrium between the production and decomposition of ozone molecules.
![]()
This ozone layer is acting like a protective layer for the life on the earth. The following reactions take place in this context:
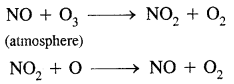
Freons are introduced in the atmosphere from aerosol sprays in which they function as propellants and from refrigerant equipment in which they act as coolants:
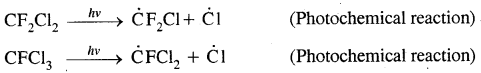
This is reactive chlorine destroys the ozone through the following reactions:
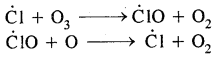
The Ozone Hole:
The depletion of the ozone layer by two major compounds [NO2 and CH4] is called the ozone hole.
In the summer season, NO2 and CH4 react with chlorine monoxide (CIO2) and chlorine atoms (Cl) forming chlorine sinks, preventing much ozone depletion.
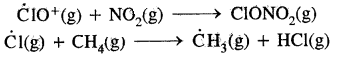
Whereas in winter, a special type of clouds, called polar stratospheric clouds are formed over Antarctica. On the surface of these clouds, chlorine nitrate (CIONO2) formed gets Hydrolysed to form hypochlorous acid. It reacts also with HCl (above) to give Cl2
CIONO2(g) + H2O(g) → HOCl(g) + HNO3(g)
CIONO2(g) + HCl(g) → Cl2(g) + HNO3(g)
When sunlight returns to Antarctica in the spring, the sum’s warmth breaks up the clouds and HOCl and Cl2 are photolysed to gaseous chlorine atoms.
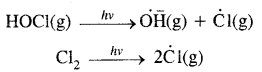
The chlorine-free radicals thus formed, initiate the chain reaction for ozone depletion. One CFC molecule destroys one lac molecules of O3.
Effects of Depletion of Ozone Layer:
- The most serious effect is the development of an ozone hole which will allow UV radiations from the sun to pass through the stratosphere and reach the earth causing among other ailments skin cancer.
- UV rays cause; damage to the cornea and lens of the eye and may cause cataract or even blindness. ,
- It affects plants chlorophyll, proteins, and causes harmful mutation.
- O3 depletion will affect the climate badly. It will upset the heat balance of the earth.
- It will lead to ecological imbalance which will adversely affect both man and animal.
Water Pollution: Major Water Pollutants
| Water Pollutants | Sources |
| 1.. Plant nutrients. | 1. Chemical fertilizer |
| 2. Sediments. | 2. Erosion of soil agriculture and strip mining. |
| 3. Microorganisms. | 3. Domestic sewage. |
| 4. Toxic heavy metals. | 4. Industries and chemical tãctories. |
| 5. Organic wastes. | 5. Domestic sewage, animal waste, decaying animals and plants and discharge from food processing factories. |
| 6. Pesticides. | 6. Chemicals used for killing insects, fungi, and weeds. |
| 7. Radioactive substances. | 7. Mining of Uranium containing minerals. |
| 8. Heat | 8. Water used for cooling in industries. |
Causes of Water Pollution:
1. Pathogens: They are disease-causing agents. They include bacteria and other organisms that enter the water from domestic sewage and animal excreta. Human excreta contain bacteria that cause gastrointestinal diseases.
2. Organic Wastes: The other major water pollutant is organic matter such as leaves, grass, trash etc. Excessive phytoplankton growth within the water is also a cause of water pollution. These are biodegradable. Decomposition of organic matter by bacteria in water causes depletion of dissolved oxygen in the water which is very essential for aquatic life.
3. Chemical Pollutants: Water-soluble inorganic chemicals like cadmium, nickel and nickel salts are an important class of water pollutants. They are dangerous to human life because our body cannot excrete them. Slowly and slowly they damage kidneys, central nervous system, liver etc. Petroleum products like oil spills in oceans, polychlorinated biphenyls (PCBs), though biodegradable are another source of organic chemicals that pollute water.
International Standards For Drinking Water:
- Fluoride: A deficiency of fluoride in water for drinking is harmful to man and causes tooth decay. Soluble fluoride is added to drinking water to bring its concentration to 1 ppm. Its concentration in water is more than 2 ppm and has harmful effects on teeth, bones.
- Lead: The upper prescribed limit for the presence of lead in water is 50 ppb. Lead damages the kidney, liver and reproductive system.
- Sulphate: Excessive sulphate (> 500 ppm) in drinking water causes a laxative effect. At moderate levels it is harmless.
- Nitrate: The maximum limit of nitrate in drinking water is 50 ppm. Excessive presence can cause disease such as methemoglobinemia (blue baby syndrome)
- Other metals: The maximum concentration of some common metals recommended in drinking water are given below:
Maximum Prescribed Concentration of Some Metals in Drinking Water:
| Metal | Maximum Concentration (ppm or mg dm-3) |
| Fe | 0.2 |
| Mn | 0.05 |
| Al | 0.2 |
| Cu | 3.0 |
| Zn | 5.0 |
| Cd | 0.005 |
Soil Pollution
Insecticides, pesticides and herbicides used for the protection of crop cause soil pollution.
Pesticides
Commonly used pesticides which are toxic substances are DDT, Aldrin, and Dieldrin. When their concentration increases beyond a limit, they cause serious metabolic and physiological disorders in higher animals. They are water-insoluble and non—biodegradable. With the passage of time, insects & pests have become immune to these pesticides and insecticides. More potent and biodegradable products like organophosphates and carbonates have beef put to use.
These chemical are extremely harmful to humans and agriculture felid work who spray them.
Nowadays, the pesticide industry is using Herbicides such as sodium chlorate (NaCIO3), sodium arsenite (Na3AsO3) and many others. But all of them are not environmental friendly. Most herbicides are toxic to mammals,
Industrial Waste:
Industrial solid wastes are sorted out as biodegradable and non- Biodegradable wastes. The former is generated by cotton mills, food processing units, paper mills and textile factories, whereas the latter is generated by thermal power plants, iron and steel plants, industries producing Al, Zn and Cu.
The disposal of non-degradable industrial solid waste should be done suitably, otherwise, it may cause a serious threat to the environment. There should be proper management of both domestic and industrial wastes.
Green Chemistry:
Green chemistry as an alternative tool for reducing pollution: One way to protect our environment from chemical effluents and waste is to use green chemistry. By green chemistry, we mean producing the chemicals of our daily needs using such reactions and chemical process which neither use toxic chemicals nor emit such chemicals into the atmosphere. Although it is a very challenging task.
Green chemistry does not employ toxic reagents and severe conditions but uses mild and environmentally friendly reagents, such as sunlight, microwaves, sound waves and enzymes. The use of sunlight and ultraviolet radiation is very useful to produce some useful products which cannot be obtained by simple chemical reactions microwaves and sound waves are used for chemical reactions giving extraordinary results which are not possible by simple chemical reactions.
Enzymes are environmentally friendly reagents. These work in aqueous solutions and at ambient temperatures. These methods are used for preparing medicines and certain antibiotics. For examples, semi-synthetic penicillins such as ampicillin, and Amoxycillin, have been prepared by using this technique.
By using these methods, green chemistry will help us to keep our environment pollution-free.
Green Chemistry in Day-to-Day Life:
1. Dry cleaning of clothes: In places of tetrachloroethene (C12C = CC12) which was earlier used for dry-cleaning nowadays liquefied carbon dioxide along with a suitable detergent is used. It will cause less harm to groundwater. Hydrogen peroxide (H2O2) is also nowadays used for bleaching clothes which gives better results.
2. Bleaching of paper: Cl2 gas which was earlier used for bleaching paper-has been replaced by hydrogen peroxide (H2O2) with a suitable catalyst.
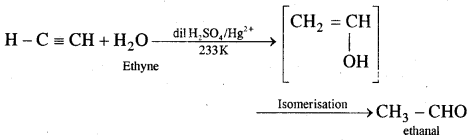
3. Synthesising chemicals: Acetaldehyde (CH3CHO) is now prepared by one-step oxidation of ethylene (H2C = CH2) in the presence of an anionic catalyst in an aqueous medium with a yield of 90% on a commercial scale.
In a nutshell, Green chemistry is a cost-effective approach, which involves
- Reduction in cost
- Reduction in energy consumption
- Reduction in waste production.