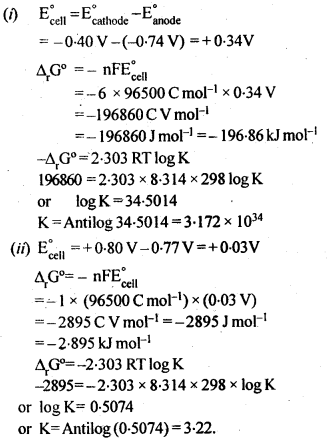
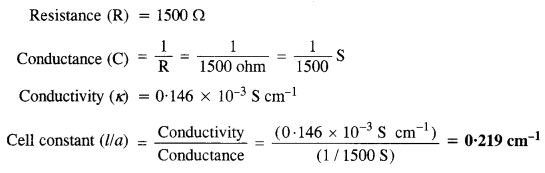
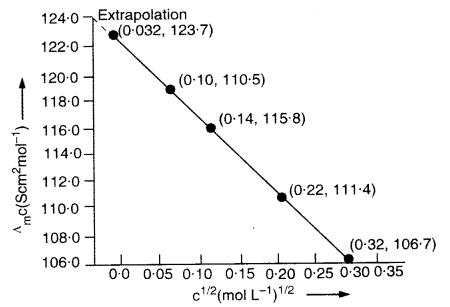
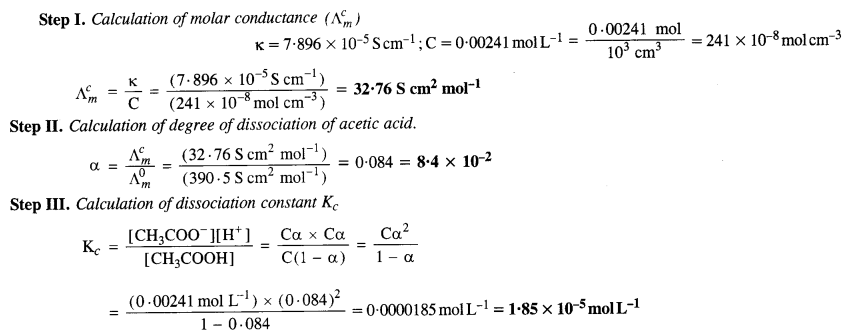
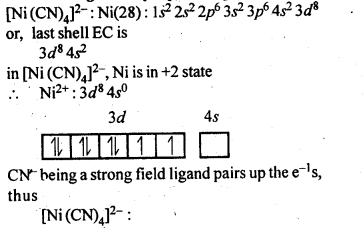
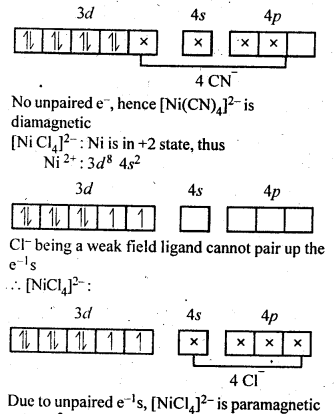
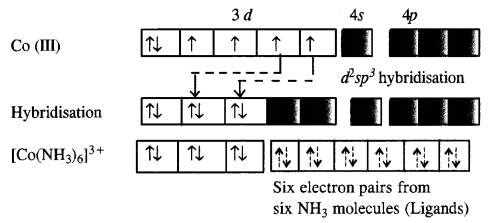
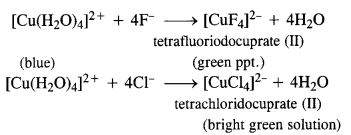
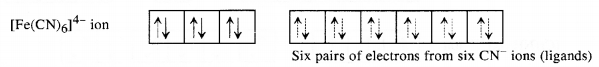
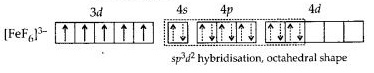
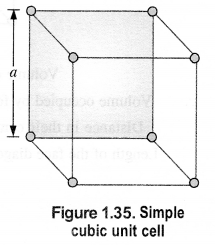
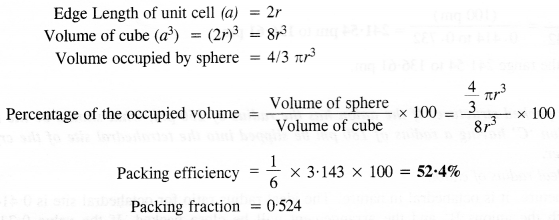
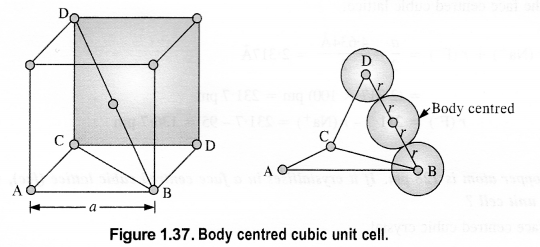
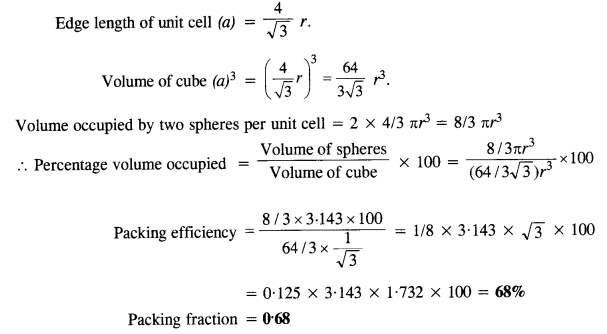
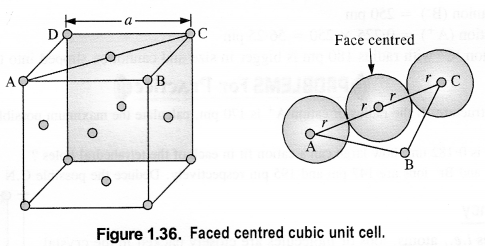
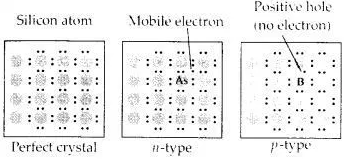
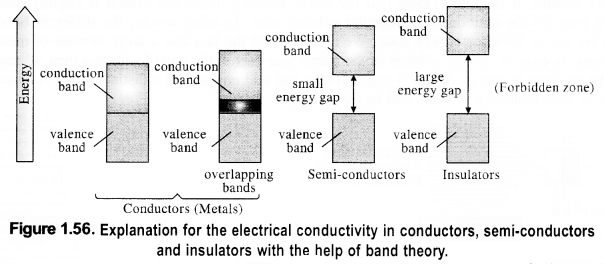
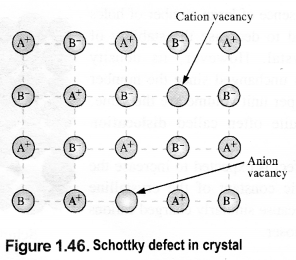
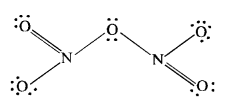
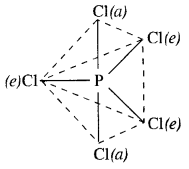
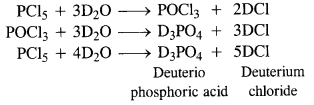
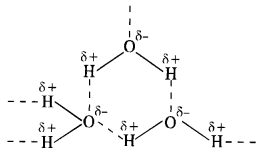
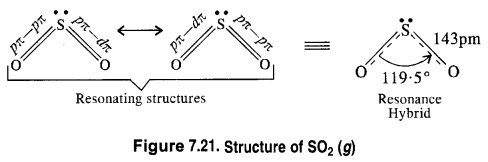
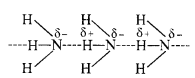
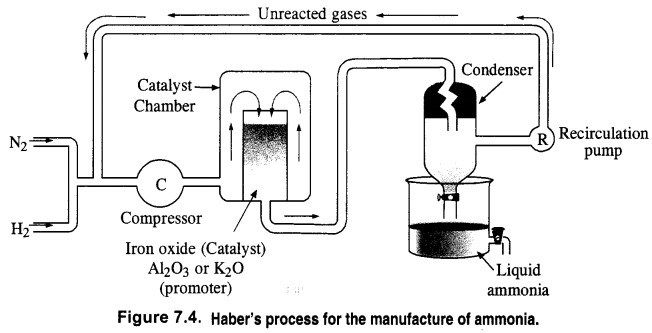
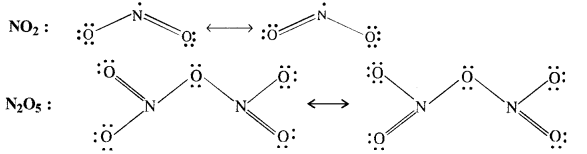
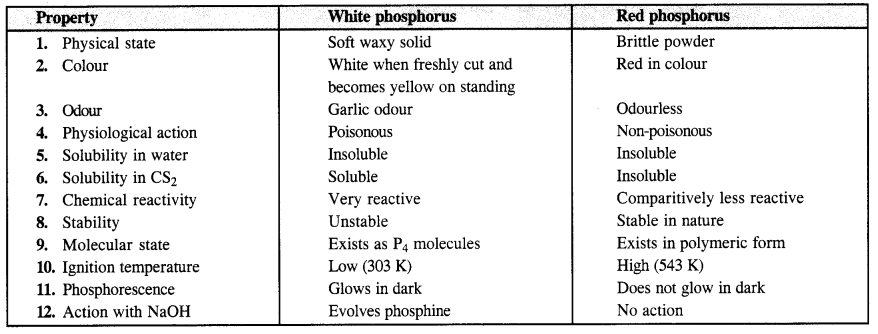
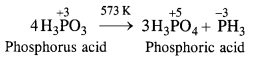
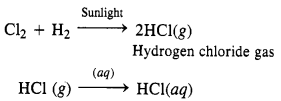
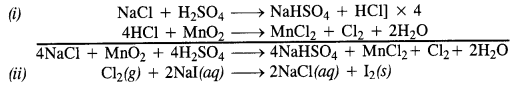
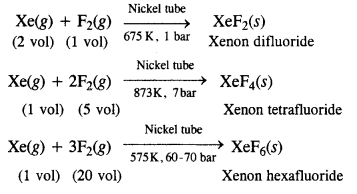
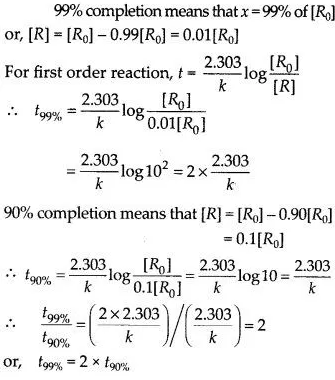
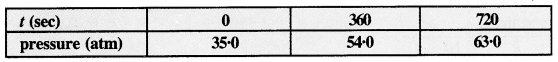
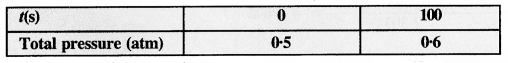
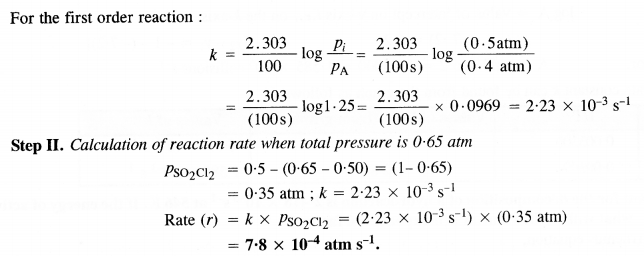
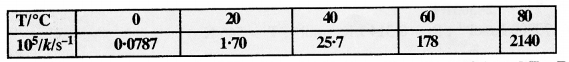
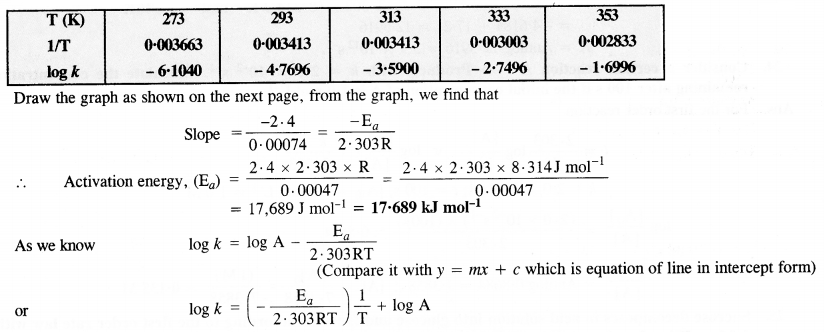
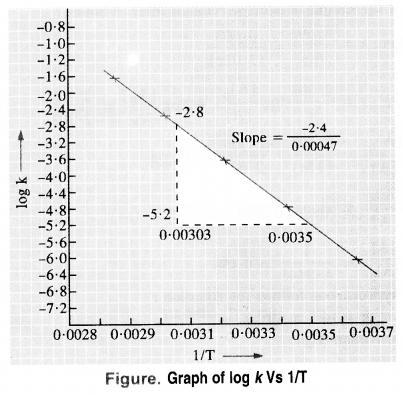
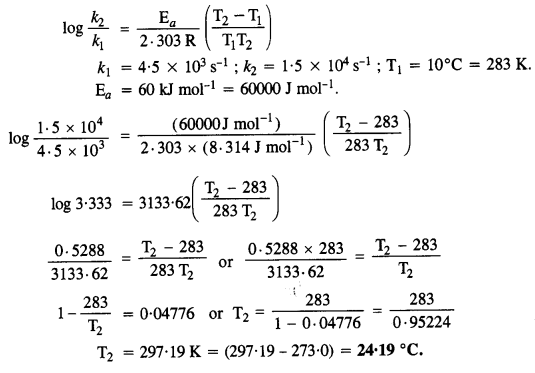
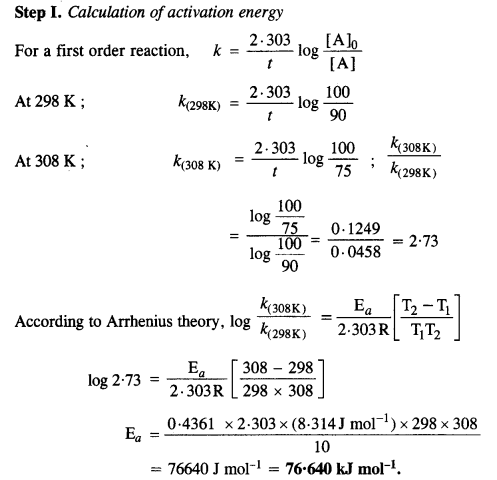
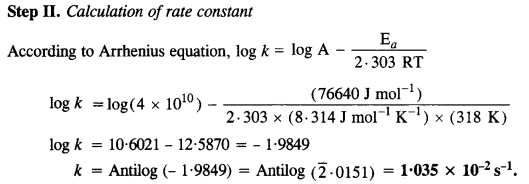
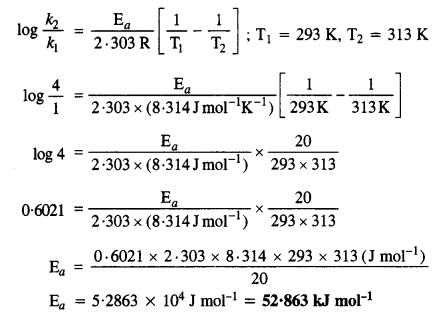
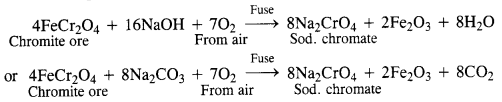
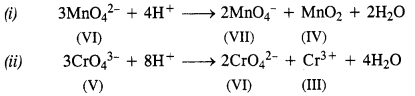
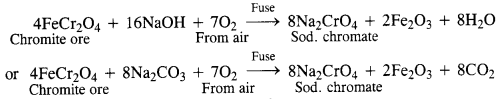
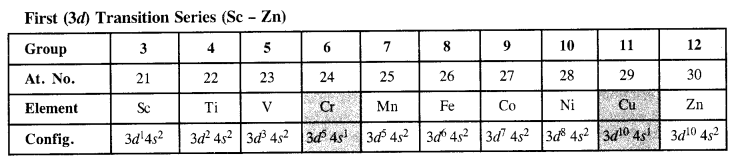
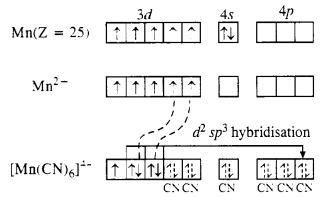
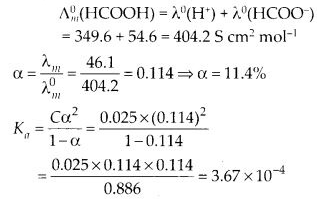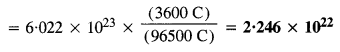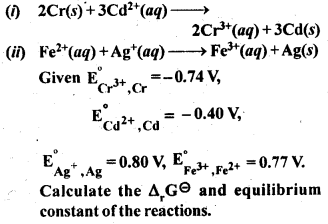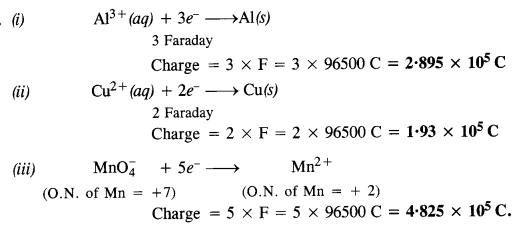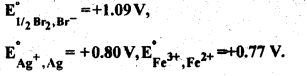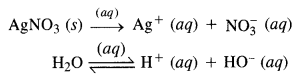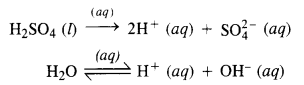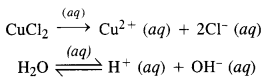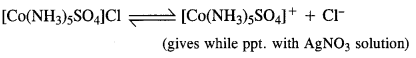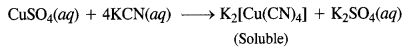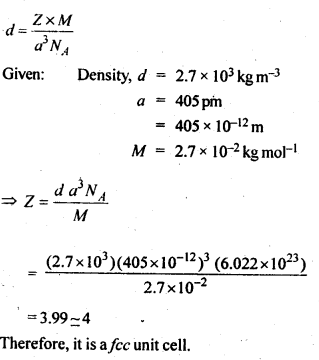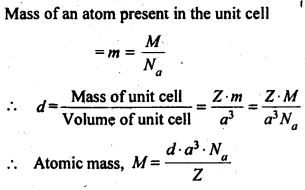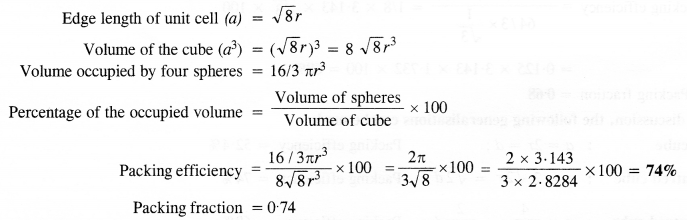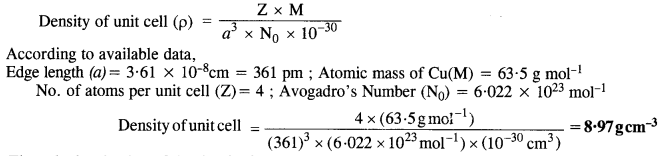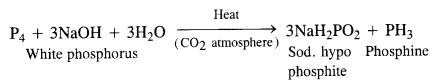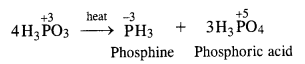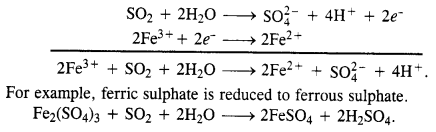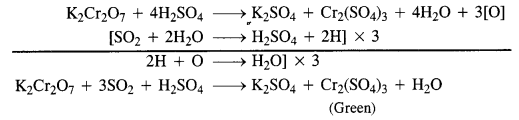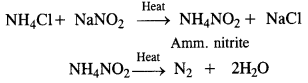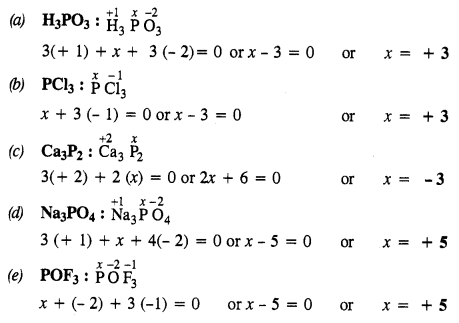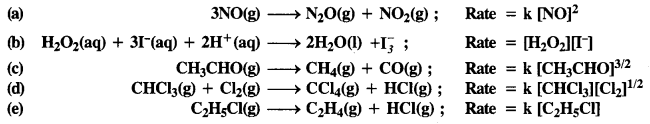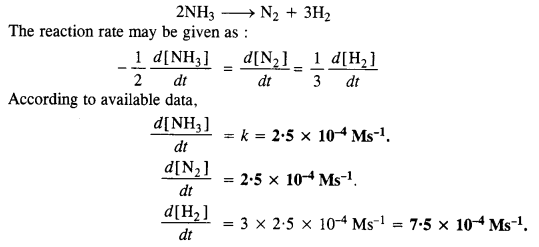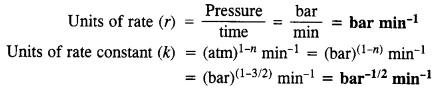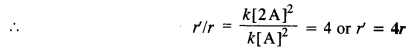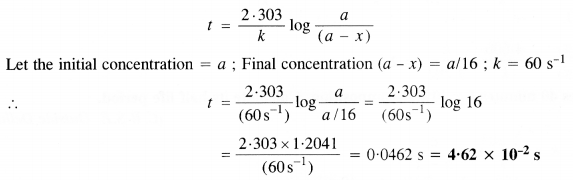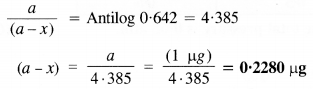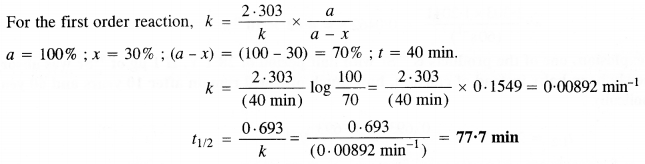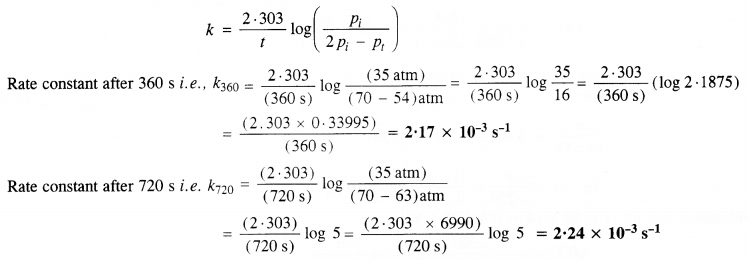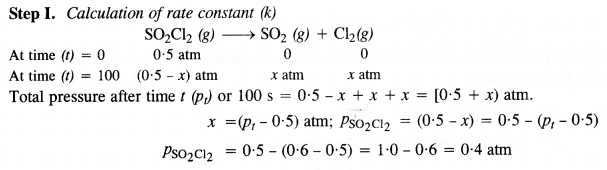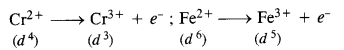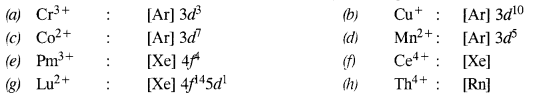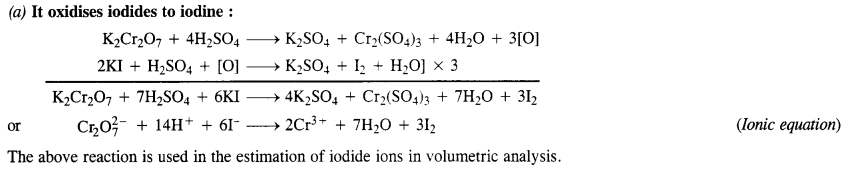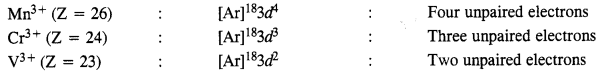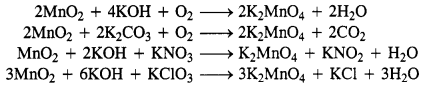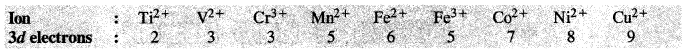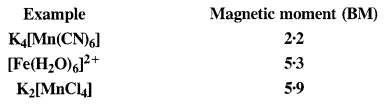Download NCERT Extra Questions for Class 9 Science: Here we are providing NCERT Extra Questions for Class 9 Science with Solutions Answers Chapter Wise Pdf free download. Students can get Class 9 Science NCERT Solutions, CBSE Class 9 Science Important Extra Questions and Answers designed by subject expert teachers.
NCERT Class 9 Science Extra Questions is the most useful study material for CBSE board students. These solved important questions are asked previously in the CBSE class 9 science board examination. Practicing with these NCERT Class 9th Science Extra Questions will improve your knowledge and problem-solving capabilities on science topics and make you feel confident in the board exams as well as competitive exams.
NCERT Extra Questions for Class 9 Science with Answers Pdf
Here is the list of CBSE NCERT Chapter Wise Extra Questions for Class 9 Science with answers and solutions designed by subject experts. Access the links & download the respective chapter extra questions & answers pdf for free.
- Matter in Our Surroundings Class 9 Extra Questions
- Is Matter Around Us Pure Class 9 Extra Questions
- Atoms and Molecules Class 9 Extra Questions
- Structure of the Atom Class 9 Extra Questions
- The Fundamental Unit of Life Class 9 Extra Questions
- Tissues Class 9 Extra Questions
- Diversity in Living Organisms Class 9 Extra Questions
- Motion Class 9 Extra Questions
- Force and Laws of Motion Class 9 Extra Questions
- Gravitation Class 9 Extra Questions
- Work, Power And Energy Class 9 Extra Questions
- Sound Class 9 Extra Questions
- Why Do we Fall Ill Class 9 Extra Questions
- Natural Resources Class 9 Extra Questions
- Improvement in Food Resources Class 9 Extra Questions
We hope the given CBSE NCERT Chapter Wise Extra Questions for Class 9 Science with answers and solutions will help you. If you have any query regarding CBSE Class 9 Science Important Extra Questions and Answers, drop a comment below and we will get back to you at the earliest.