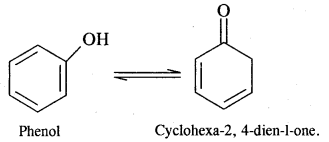
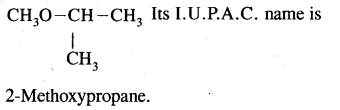
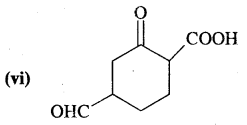
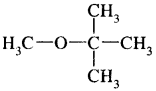
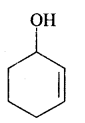
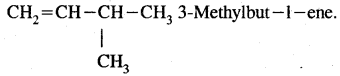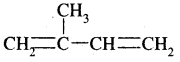We have decided to create the most comprehensive English Summary that will help students with learning and understanding.
Keeping Quiet Poem Summary in English by Pablo Neruda
Keeping Quiet Poem by Pablo Neruda About the Poet
Pablo Neruda, was a Nobel Prize-winning poet. He adopted this pen name in 1920, in the memory of Czechoslovak poet, Jan Neruda.
Neruda wrote in a variety of styles, such as love poems as in his collection, Twenty Poems of Love and , a Song of Despair, surrealist poems, historical epics, and overtly political manifestos. He travelled the ? world for many years, and returned to Chile in 1943. In 1945, he joined the Communist Party of Chile and became Senator of the Republic. In 1971, Neruda won the Nobel Prize for Literature.
Pablo Neruda I remained active in the literary and political arena even before his death. His poetry bears an impact on his political activities and expresses his experiences in repression during his exile. He wrote over 140 poems. Colombian novelist Gabriel Garda Marquez once called him “the greatest poet of the 20th § century in any language”.
| Poet Name | Pablo Neruda |
| Born | 12 July 1904, Parral, Chile |
| Died | 23 September 1973, Santiago, Chile |
| Full Name | Ricardo Eliecer Neftali Reyes Basoalto |
| Awards | Nobel Prize in Literature, Lenin Peace Prize |
| Spouse | Matilde Urrutia (m. 1966–1973) |

Keeping Quiet Poem Introduction to the Poem
In this poem, the poet aims to appeal to the readers to take some time out of their busy life for a little introspection and retrospection. The title, “Keeping Quiet” is symbolic of stopping all the activities and keep the mind quiet by not doing anything, but to question and understand the purpose of the world that humans have created around themselves.
Keeping Quiet Poem Theme
In the poem, the poet advocates to keep quiet and still for a while to introspect and understand ourselves and our relationships, and to build a peaceful and harmonious world over. He asks all the human beings to stop all worldly activities for a while and spend few quiet moments in introspection.
Keeping Quiet Poem Summary in English
In this poem, the poet has emphasised the need to introspect and bring in the spirit of brotherhood among the people of the world. He wants people to stop talking and stop all movements symbolising agitation and restlessness till he counts twelve, that is, a short period of time. These moments of silence would be strange and exotic because in our mundane life, we are working towards achieving selfish goals, regardless of the others’ requirements and emotions. Hence, this sudden silence would give us an opportunity to introspect. Since we would not speak for a while, barriers between communities would break and a sense of brotherhood would prevail.
Man would get an opportunity to realise how he is destroying nature and how he is harming himself. Futile wars against man and nature would be arrested and a new feeling of unity would be experienced. The poet does not want his desire for inactivity to be misunderstood as a state of uselessness or in action. He wants man to learn a lesson from the earth. The earth appears to be inactive, yet it is selflessly productive. Man too could be productive and progressive without any aggression, selfishness and the urge for destruction.
Short Summary Of Keeping Quiet Reference-to-Context Questions
Read the extracts given below and answer the questions that follow.
1. Now we will count to twelve
and we will all keep still.
For once on the face of the Earth
let’s not speak in any language,
let’s stop for one second,
and not move our arms so much.
a. What is the significance of the number ‘twelve’ ?
Answer:
The number ‘twelve’ signifies the twelve divisions of time. It is a measure of time.
b. Which two activities does the poet want us to stop?
Answer:
The poet wants us to keep quiet and not speak. He does not want us to move our arms.
c. What does the poet mean by ‘let’s not speak in any language’?
Answer:
The poet pleads us to be silent and introspect.
d. Describe the pun in the word, ‘arms’.
Answer:
‘Arms’ refers to weapons and the arms in the human body.
2. It would be an exotic moment
without rush, without engines,
we would all be together
in a sudden strangeness.
a. What does ‘It’ refer to?
Answer:
‘It’ refers to the moment when there is peace and quietness which the poet considers would be exotic.
b. Who is the poet addressing to?
Answer:
The poet is addressing his readers.
c. What would be the moment like?
Answer:
The absence of hustle and bustle of life would create a feeling of peace and quiet which would make us united.
d. What does ‘exotic’ mean?
Answer:
It means ‘unique’ as the moment would be mysteriously different from the usually prevailing situation.
3. Fishermen in the cold sea,
would not harm whales
and the man gathering salt
would look at his hurt hands.
a. What are ‘fishermen’ symbolic of?
Answer:
‘Fishermen’ symbolise proficient hunters of all kinds. These people have been indiscriminately exploiting nature and Mother Earth for their own vested interests.
b. What message does the poet seem to give in these lines?
Answer:
The message that Neruda wishes to convey is that the desire of man for more and more progress and advancement has done more destruction than development. Man seems to have no concern and care for his brethren.
c. What image does Neruda portray in the last lines?
Answer:
The image that Neruda seems to create in the last lines is one of the incessant sufferings. In order to make life more comfortable, he pays no heed to the sufferings he has to undergo. The men, who gather salt do not look at their hurt palms because they are more busy making life more comfortable for themselves and for their family.
d. What basic idea does the poet empahsise upon?
Answer:
It is the environmental protection through man’s introspection and action.
4. Those who prepare green wars,
wars with gas, wars with fire,
victory with no survivors,
would put on clean clothes
and-walk about with their
brothers
in the shade, doing nothing.
a. Who are ‘those’ here?
Answer:
Here, ‘those’ are the politicians, scientists, statesmen and those overambitious powers to be, who in their zeal for dominance are involved in initiating and aggravating wars.
b. What are ‘green wars, wars with gas, wars with fire’?
Answer:
These are the nuclear and chemical weapons which have been created by man and used in waging wars against countries. In fact, these wars have brought mankind to its fatal end. These wars are also against the environment.
c. What does Neruda mean by ‘victory with no survivors’?
Answer:
The victory that may be gained with the help of nuclear and chemical warfare would actually leave no one alive in that area. There is no point of winning such a war which cannot be savoured as there would be no survivors.
d. Which poetic device is used in the third line of the extract?
Answer:
It is Paradox as opposite ideas are explained by this phrase.
5. What I want should not be
confused
with total inactivity.
Life is what it is about;
I want no truck with death.
a. What does Neruda imply by ‘total inactivity’?
Answer:
‘Total inactivity’ would imply that mankind should channelise his activities towards construction and he should put an end to his destructive activities. It certainly does not mean no action because life is an on-going process.
b. What is life about?
Answer:
Life cannot be brought to a standstill under any circumstances. It is an on-going process and it has to move on.
c. What does Neruda mean when he says, ‘I want no truck with death’?
Answer:
Neruda says that when he talks of counting upto twelve, meditating and introspecting, he is not advocating death-like silence. To bring all activities to a halt is only to facilitate introspection, and not to bring life to a standstill. Life is an on-going process and humanity should move on.
d. What is the underlying thought of the poet here?
Answer:
We should not act physically but we must introspect.
6. If we were not so single-minded
about keeping our lives moving,
and for once could do nothing,
perhaps a huge silence
might interrupt this sadness
of never understanding ourselves
and of threatening ourselves with
death.
a. What is man ‘single-minded’ about?
Answer:
Man is single-minded about his own progress and advancement. He is so focussed on his own development that he forgets to visualise the pros and cons of reckless development. The consequences and impact of materialistic progress should be taken into consideration.
b. Explain, ‘sadness of never understanding ourselves’.
Answer:
Mankind, in its race for materialistic success has sacrificed his emotional needs at some point of time. People are so engrossed in fulfilling their ambitions and successfully completing their duties that they tend to forego their inner mental satisfaction. Thus, they tend to become sad and isolated.
c. How has mankind threatened itself with death?
Answer:
The race for more arms and ammunitions and the desire to overpower and dominate over more and more areas and countries is what has threatened mankind with death. The mass destruction of nature is also one main reason for large-scale death of humanity.
d. What does ‘keeping our life moving’ mean?
Answer:
It means making progress without a second thought.
7. Perhaps the Earth can teach us
as when everything seems dead
and later proves to be alive.
Now I’ll count up to twelve
and you keep quiet and I will go.
a. What does the earth teach us?
Answer:
The earth teaches us how new life springs from dead remains and how there is life under apparent stillness.
b. What does the poet mean to achieve by counting upto twelve?
Answer:
The poet wants to achieve peace by counting upto twelve. He wants us to introspect in a moment of silence.
c. What is the significance of ‘keeping quiet’?
Answer:
Keeping quiet doesn’t mean being idle. It means that we should avoid all such activities, which are hurting the nature, and in turn, us.
d. What is the poetic device used in the first line?
Answer:
Personification because earth is depicted like an instructor to us.