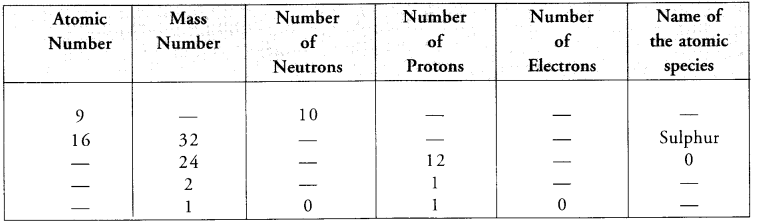
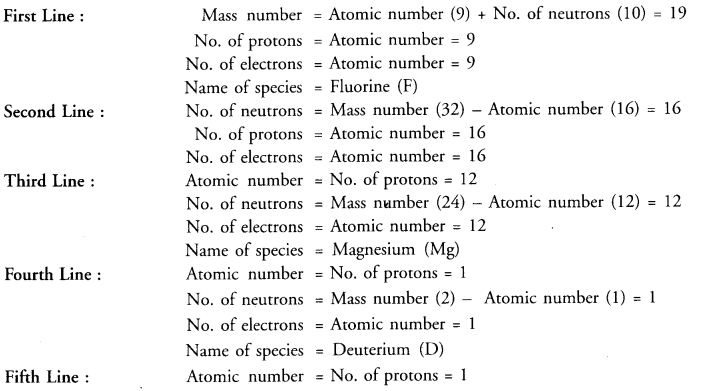
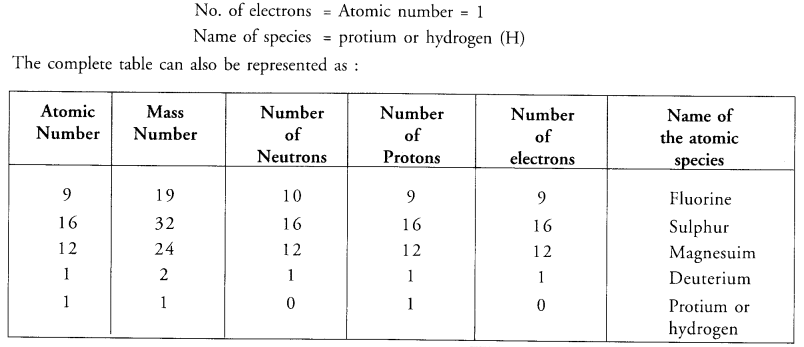
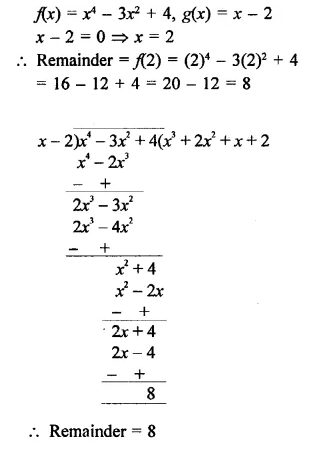
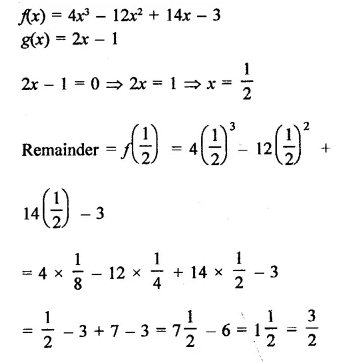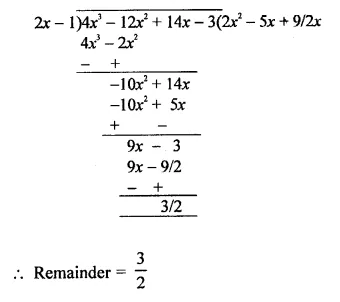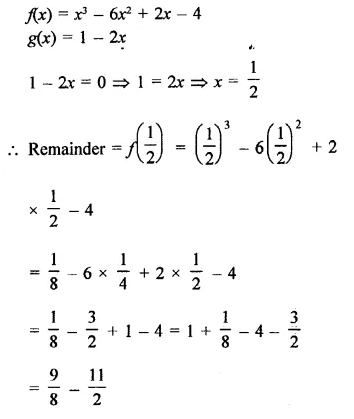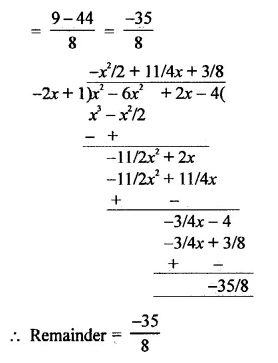Value Based Questions in Science for Class 9 Chapter 2 Is Matter Around Us Pure
These Solutions are part of Value Based Questions in Science for Class 9. Here we have given Value Based Questions in Science for Class 9 Chapter 2 Is Matter Around Us Pure
Question 1.
A student was asked by his teacher to separate an impure sample of sulphur containing sand as the impurity. He tried to purify it with the help of sublimation. But he was not successful. Particles of sulphur could not be separated completely from sand.
- Why did not the sublimation process succeed ?
- Suggest an alternate method to affect the separation.
- What is the value based information associated with this ?
Answer:
- Sulphur is not of volatile nature. Upon heating, it melts. Therefore, sublimation process could not succeed.
- The student should have dissolved the impure sample in carbon disulphide. It is a liquid in which sulphur completely dissolves while iron does not. From the solution, sulphur can be recovered with the help of crystallization process.
- The process of sublimation is useful only if one of the constituents present in the mixture can sublime while the others do not undergo sublimation.
More Resources
- Value Based Questions in Science for Class 9
- HOTS Questions for Class 9 Science
- NCERT Solutions for Class 9 Science
- NCERT Exemplar Solutions for Class 9 Science
- Previous Year Question Papers for CBSE Class 9 Science
Question 2:
Amit was asked by his teacher to separate a liquid mixture of acetone and ethyl alcohol. He set up a distillation apparatus and tried to distil the mixture. To his surprise, both the liquids got distilled. Teacher told Amit to repeat the experiment by using a fractionating column in the distillation flask. Amit followed the advice of the teacher and he was able to separate the two liquids.
- Why was Amit not successful in separating the liquid mixture earlier ?
- Why did teacher ask him to use the fractionating column ?
- Which liquid was distilled first ?
- As a student of chemistry, what value based information you have gathered ?
Answer:
- The difference in boiling point temperatures of acetone (56°C) and ethyl alcohol (78°C) is only 22°C. Therefore, process of simple distillation fails in this case.
- Fractionating column is quite effective in this case because it obstructs the distillation of ethyl alcohol which is high boiling and at the same time helps in the distillation of acetone which is low boiling.
- Acetone was distilled first since it has comparatively low boiling point.
- The process of simple distillation can be used only in case, the liquids present in the mixture differ in their boiling point by 25°C or more.
Question 3.
A housewife got a cut on her finger while working in the Kitchen. She tried to stop the bleeding by applying dettol on it but it was not effective. By chance, her friend was also there. She asked her to rub alum on the cut which she did. The bleeding immediately stopped.
- Why was not dettol effective in stopping bleeding ?
- Why was alum effective ?
- What valuable service was done by the friend to the housewife ?
Answer:
- Blood is a colloidal solution and the colloidal particles of RBCs carry charge. Dettol is an organic compound. It could not neutralise the charge on the colloidal particles. Therefore, bleeding did not stop.
- Alum is a salt in which charged ions are present. When alum was applied on the cut, the charged ions neutralised the charge on the colloidal particles. In the absence of charge, blood became thick and bleeding stopped. In otherwords, blood got coagulated.
- The friend has a proper knowledge of colloidal solutions. She must be a student of chemistry. Timely help by her stopped the bleeding. Otherwise, there would have been a further loss of blood.
Question 4.
Mallika’s mother was suffering from cold and cough. Mallika prepared tea for her mother. She boiled water in a pan, then she added tea leaves, sugar and milk to it. She filtered the tea in a cup and served to her mother.
- Explain the values shown by Mallika.
- Identify solute, solvent, residue and filtrate in this activity. (CBSE 2013)
Answer:
- Mallika used the knowledge of chemistry to provide relief to her mother. Actually she prepared an extract of tea leaves which is helpful in curing cold and cough and gives warmth to the body.
- Solute : tea leaves and sugar ; Solvent : water and milk; Filtrate : homogeneous mixture of water, milk, sugar and extract of tea leaves.
Question 5.
Nikhil’s father was suffering from high blood pressure and cardiac problems. Doctor suggested him to take low fat milk. Nikhil churned the milk and separated the butter (fat) from it and then he served that milk to his father. Answer the following questions based on above information :
- Name the technique by which Nikhil separated butter from milk.
- Write any other application of that technique.
- Which values are reflected in Nikhil’s behaviour ?
Answer:
- It is called centrifugation carried in a centrifugal machine.
- Washing machines that are used for washing dirty clothes are centrifugal machines.
- Nikhil had the proper knowledge of chemistry. He used this to separate fat from milk with the help of the centrifugal machine. In this way, he rendered proper service to his father.
Hope given Value Based Questions in Science for Class 9 Chapter 2 Is Matter Around Us Pure are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.