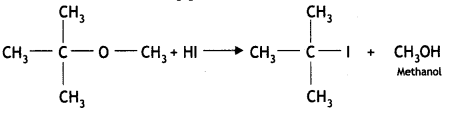
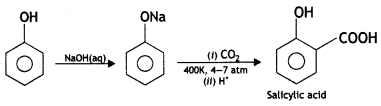
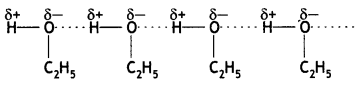
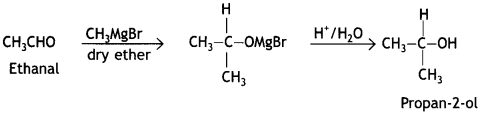
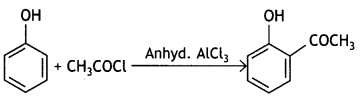
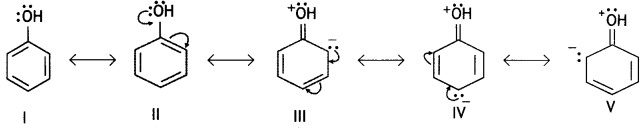
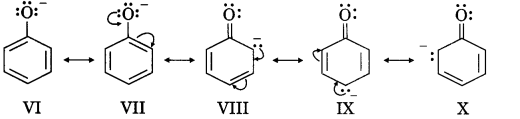
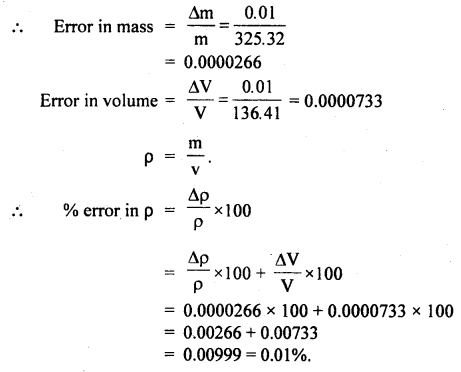
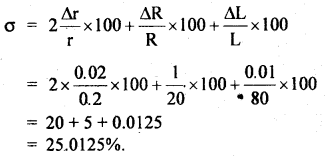
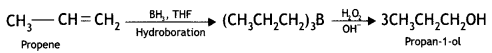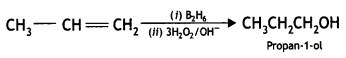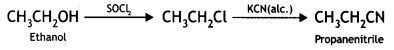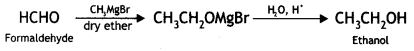Students can access the CBSE Sample Papers for Class 10 Sanskrit with Solutions and marking scheme Set 3 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Sanskrit Set 3 with Solutions
समयः- होरात्रयम्
सम्पूर्णाङ्काः – 80
सामान्यनिर्देशाः
- कृपया सम्यक्तया परीक्षणं कुर्वन्तु यत् अस्मिन् प्रश्नपत्रे 19 प्रश्नाः सन्ति।
- उत्तरलेखनात् पूर्व प्रश्नस्य क्रमाङ्कः अवश्यं लेखनीयः।
- अस्य प्रश्नपत्रस्य पठनाय 15 निमेषाः निर्धारिताः सन्ति। अस्मिन् अवधौ केवलं प्रश्नपत्रं पठनीयम् उत्तरपुस्तिकायां च किमपि न लेखनीयम्।
प्रश्नपत्रस्वरूपम्
‘अ’-भागः (बहुविकल्पात्मकः) 40 अङ्काः
‘आ’-भागः (वर्णनात्मकः) 40 अङ्काः
(i) अस्मिन् प्रश्नपत्रे द्वौ भागौ स्तः।
(ii) ‘अ’- भागः बहुविकल्पात्मकः अस्ति।
(iii) ‘आ’-भागः वर्णनात्मकः अस्ति।
(iv) प्रश्नसङ्ख्या प्रश्नपत्रानुसारम् अवश्यमेव लेखनीया।
(v) सर्वेषां प्रश्नानाम् उत्तराणि संस्कृतेन लेखनीयानि।
(vi) प्रश्नानां निर्देशाः ध्यानेन अवश्यं पठनीयाः।
‘अ’-भागः- बहुविकल्पात्मकाः प्रश्नाः (अङ्काः 40)
अनुप्रयुक्त-व्याकरणम् (अङ्काः 25)
प्रश्न 1.
अधोलिखितवाक्येषु रेखाङ्कितपदस्य सन्धिपदं सन्धिच्छेदपदं वा चिनुत-(केवलं प्रश्नचतुष्टयम्) (1 x 4 = 4)
(i) रामः + अत्र भोजनं करोति।
(क) रामश्त्र
(ख) रामोत्रं
(ग) रामोऽत्र
उत्तर
(ग) रामोऽत्र
(ii) एतस्मादेव पाठात् त्वं पठ।
(क) एतत् + अस्मादेव
(ख) एतस्मात् + एव
(ग) एतत् + अस्मादेव
उत्तर
(ख) एतस्मात् + एव
(iii) सम्यक् + नेता एव समाजस्य उद्धारकः भवति।
(क) सम्यवनेता
(ख) सम्यङ्नेता
(ग) सम्यक्नेता
उत्तर
ख) सम्यङ्नेता
(iv) हे ईवश्र! सत् + सतिं यच्छ।
(क) सन्मतिं
(ख) सच्मतिं
(ग) सत्मतिं
उत्तर
(क) सन्मतिं
(v) अहम् नमस्तुभ्यम्।
(क) नमस् + तुभ्यम्
(ख) नमः + तुभ्यम्
(ग) नमस्तु + भ्यम्
उत्तर
(ख) नमः + तुभ्यम्
प्रश्न 2.
अधोलिखितवाक्येषु रेखाङ्कितपदानां समासं विग्रहं वा प्रदत्तविकल्पेभ्यः चिनुत। (केवलं प्रश्नचतुष्टयम्) (1 x 4 = 4)
(i) मातापितरौ सदैव वन्दनीयौ।
(क) मातरौ पितरौ च
(ख) माता च पितृ च
(ग) माता च पिता च
उत्तर
(ग) माता च पिता च
(ii) रामः कानने अनुमृगम् धावति।
(क) मृगम् अनतिक्रम्य
(ख) मृगस्य पश्चात्
(ग) मृगस्य योग्यम्
उत्तर
(ख) मृगस्य पश्चात्
(iii) साधुवृत्तिम् समाचरेत्।
(क) साधुम् वृत्तिम्
(ख) साधोः वृत्तिम्
(ग) साधौ वृत्तिम्
उत्तर
(ख) साधोः वृत्तिम्
(iv) समयम् अनतिक्रम्य कार्यम् कुरु।
(क) यथासमयः
(ख) यथासमयम्
(ग) समयंयथा
उत्तर
(ख) यथासमयम्
(v) सिंहः सक्रोधम् अवदत्।
(क) क्रोधम् सहितम्
(ख) क्रोधात् सहितम्
(ग) क्रोधेन सहितम्
उत्तर
(ग) क्रोधेन सहितम्
प्रश्न 3.
अधोलिखितवाक्येषु रेखातिपदानां प्रकृति-प्रत्ययौ संयोज्य विभज्य वा उचितम् उत्तरं विकल्पेभ्यः चिनुत। (केवलं प्रश्नचतुष्टयम्) (1 x 4 = 4)
(i) यत्रास्ते सा धूर्ता तत्र गम्यताम्।
(क) धूर्त + आ
(ख) धूर्त + ङीप्
(ग) धूर्त + टाप्
उत्तर
(ग) धूर्त + टाप्
(ii) कस्मिन् जने ईश्वर + स्व भवति?
(क) ईश्वतत्वं
(ख) ईश्वरत्वम्
(ग) ईश्वरतन्तः
उत्तर
(ख) ईश्वरत्वम्
अथवा
कालिदासस्य कवित्वम् श्लाघनीयं वर्तते।
(क) कवि + त्व
(ख) कवी + त्व
(ग) कवि + तल्
उत्तर
(क) कवि + त्व
(iii) धैर्यवन्तः जनाः एव सफलतां लभन्ते।
(क) धैर्य + क्तवतु
(ख) धैर्य + शानच्
(ग) धैर्य + मतुप्
उत्तर
(ग) धैर्य + मतुप्
(iv) विद्वांसः एव लोकेऽस्मिन् चक्षुष् + मतुप प्रकीर्तिताः।
(क) चक्षुमन्तौ
(ख) चक्षुष्मन्तः
(ग) चक्षुमान्
उत्तर
(ख) चक्षुष्मन्तः
प्रश्न 4.
वाच्यस्य नियमानुगुणम् उचितं विकल्पं चिनुत। (केवलं प्रश्नत्रयम्) (1 x 3 = 3)
(i) सुरभिः – किं त्वं अद्य समाचार पत्रे समाचारान्
(क) पठति
(ख) पठसि
(ग) पठामि
उत्तर
(ख) पठसि
(ii) सुगन्धा- आम् मया अद्य …….पठयन्ते।
(क) समाचारम्
(ख) समाचारान्
(ग) समाचाराः
उत्तर
(ग) समाचाराः
(iii) सुरभि:- ………………. अपि प्रतिदिनं समाचारं पठामि।
(क) त्वम्
(ख) सः
(ग) अहम्
उत्तर
(ग) अहम्
(iv) सुगन्धा – ……………….. तु दूरदर्शने अपि दृश्यते।
(क) त्वम्
(ख) मया
(ग) अहम्
उत्तर
(ख) मया
प्रश्न 5.
प्रदत्तेभ्यः विकल्पेभ्यः समुचितं कालबोधकशब्दं चिनुत- (केवलं प्रश्नचतुष्टयम्) (1 x 4 = 4)
(i) अहम् सायंकाले 4:30 ………………. वादने खेलामि।
(क) सार्ध-चतुर्
(ख) सपाद-चतुर्
(ग) चतुर्-सार्ध
उत्तर
(क) सार्ध-चतुर्
(ii) 6:00 ……………………. वादने क्रीडाक्षेत्रात् गृहं आगच्छामि।
(क) नव
(ख) षड्
(ग) सार्ध
उत्तर
(ख) षड्
(iii) 8:15 …………………… वादने भोजनं करोमि।
(क) सार्ध-अष्ट
(ख) सपाद-अष्ट
(ग) सार्ध-सप्त
उत्तर
(ख) सपाद-अष्ट
(iv) 9:00 ……………………… वादनं पर्यन्तम् पठामि।
(क) नव
(ख) अष्ट
(ग) सपाद-नव|
उत्तर
(क) नव
(v) 10:45 …… …… वादने शयनं करोमि।
(क) सपाद-दश
(ख) पादोन-एकादश
(ग) पादोन-दश
उत्तर
(ख) पादोन-एकादश
प्रश्न 6.
वाक्यानुगुणम् उचिताव्ययपदं चिनुत- (केवलं प्रश्नत्रयम्) (1 x 3 = 3)
(i) मनुष्यः अनिच्छन् …………….. पापं करोति।
(क) सहसा
(ख) अपि
(ग) वृथा
उत्तर
(ख) अपि
(i) प्रायः बालशिशुः ….. चलति।
(क) विना
(ख) उच्चैः
(ग) शनैः
उत्तर
(ग) शनैः
(iii) मेघाः आकाशे ………….. गर्जन्ति।
(क) उच्चैः
(ख) शनैः
(ग) नीचैः
उत्तर
(क) उच्चैः
(v) ………………. क्रियाम् न विदधीत
(क) अधुना
(ख) विना
(ग) सहसा
उत्तर
(ग) सहसा
प्रश्न 7.
अधोलिखितवाक्येषु रेखाङ्कितपदम् अशुद्धम् अस्ति। शुद्धं पदं विकल्पेभ्यः चिनुत- (केवलं प्रश्नत्रयम्) (1 x 3 = 3)
(i) भवान् कुत्र गच्छसि?
(क) गच्छति
(ख) गच्छन्ति
(ग) गच्छामि
उत्तर
(क) गच्छति
(ii) त्वं मम मित्रम् अस्ति
(क) सन्ति
(ख) असि
(ग) आसीत्
उत्तर
(ख) असि
(iii) वृक्षे फलाः सन्ति।
(क) फले
(ख) फलानि
(ग) फलम्
उत्तर
(ख) फलानि
(iv) ता: बालिका गीतं गायन्ति।
(क) बालकाः
(ख) बालिकाम्
(ग) बालिकाः
उत्तर
(ग) बालिकाः
पठितावबोधनम् (अङ्काः 15)
प्रश्न 8.
रेखाङ्कितपदानि आधृत्य समुचितं प्रश्नवाचकपदं चिनुत। (केवलं प्रश्नपञ्चकम् ) (1 x 5 = 5)
(i) मयूरस्य नृत्यं प्रकृतेः आराधना।
(क) कस्य
(ख) कस्याः
(ग) कस्याम्
उत्तर
(ख) कस्याः
(ii) सः ऋषभः हलमूढ्वागन्तं अशक्तः क्षेत्रे पपात।
(क) कुत्र
(ख) कदा
(ग) किमर्थम
उत्तर
(क) कुत्र
(iii) शकटीयानम् कज्जलमलिनं घूमं मुञ्चति।
(क) कीदृशः
(ख) कम्
(ग) किम्
उत्तर
(ग) किम्
(iv) इति उक्त्वा आरक्षी उच्चैः अहसत्।
(क) कैः
(ख) कथम्
(ग) किमर्थम्
उत्तर
(ख) कथम्
(v) सः भारवेदनया क्रन्दति स्म।
(क) केन
(ख) कया
(ग) कस्मै
उत्तर
(ख) कया
(vi) मृगाः मृगैः सह अनुव्रजन्ति।
(क) काः
(ख) कौ
(ग) के
उत्तर
(ग) के
प्रश्न 9.
अधोलिखितवाक्येषु रेखाङ्कितपदानां प्रसङ्गानुकुलम् उचितार्थं चिनुत- (केवलं प्रश्नचतुष्टयम्) (1 x 4 = 4)
(i) क्रुद्धः सिंहः उच्चैः अगर्जत्।
(क) कुपितः
(ख) हसितः
(ग) भीत:
उत्तर
(क) कुपितः
(ii) अहम् अनृतम् न वदामि।
(क) नृतम्
(ख) सत्यम्
(ग) असत्यम्
उत्तर
(ग) असत्यम्
(iii) विद्यालये सर्वतः छात्रैव छात्राः सन्ति।
(क) समन्ततः
(ख) परितः
(ग) उभयतः
उत्तर
(क) समन्ततः
(iv) सैनिकाः देशस्य शत्रून् मारयन्ति।
(क) हितैषिणम्
(ख) अरीन्
(ग) उरगान्
उत्तर
(ख) अरीन्
(v) पुरा भोजनामकः नृपः आसीत्।
(क) अधुना
(ख) प्राचीनकाले
(ग) एकदा
उत्तर
(ख) प्राचीनकाले
प्रश्न 10.
भाषिककार्यसम्बद्धानां प्रश्नानां समुचितम् उत्तरं विकल्पेभ्यः चिनुत-(केवलं प्रश्नषट्कम्) (1 x 6 = 6)
(i) गच्छन्तम् पदस्य विपर्ययपदं किम्?
(क) गच्छताम्
(ख) आगच्छन्तम्
(ग) आगच्छ
उत्तर
(ख) आगच्छन्तम्
(ii) “प्रथमः धर्मः’ इति अनयोः पदयोः विशेष्यपदं किम्?
(क) प्रथमः
(ख) धर्मः
(ग) धर्म:प्रथमः
उत्तर
(ख) धर्मः
(iii) सः बसयानम् ‘विहाय’ पदातिरेव प्राचलत्। अत्र विहाय पदाय किम् पदम् प्रयुक्तम्?
(क) विनियोग
(ख) गत्वा
(ग) परित्यज्य
उत्तर
(ग) परित्यज्य
(iv) “पिताऽस्य किं तपस्तेपे इत्युक्तिस्तत्कृतज्ञता” अत्र अस्य पदं कस्मै प्रयुक्तम्?
(क) पित्रे
(ख) पुत्राय
(ग) शिष्याय
उत्तर
(ख) पुत्राय
(v) न्यायधीशः तम् शवं न्यायालये आनेतुम् आदिष्टवान्। अत्र ‘अदिष्टवान्‘ क्रिया पदस्य कर्तृपदम किम्?
(क) तम्
(ख) शवम्
(ग) न्यायधीशः
उत्तर
(ग) न्यायधीशः
(vi) पक्षिणः हर्षमिश्रितं कलरवं कुर्वन्ति’ अत्र क्रियापदं किम्?
(क) कलरवं
(ख) कुर्वन्ति
(ग) पक्षिणः
उत्तर
(ख) कुर्वन्ति
(vii) कृषक: इति कर्तृपदस्य क्रियापदम् किम् अस्ति?
(क) तम्
(ख) बहुधा
(ग) पीडयति
उत्तर
(ग) पीडयति
(viii) भयङ्करा व्याघ्रमारी तत्र अस्ति। अत्र विशेषणपदं किम्?
(क) व्याघ्रमारी
(ख) भयङ्करा
(ग) तत्र
उत्तर
(ख) भयङ्करा
‘आ’-भाग:- वर्णनात्मकाः प्रश्नाः (अङ्काः 40)
अपठितावबोधनम् (अङ्काः 10)
प्रश्न 11.
अधोलिखितं गद्यांशं पठित्वा प्रदत्तप्रश्नानाम् उत्तराणि संस्कृतेन लिखत। (10)
वर्तमानकाले मानवः समस्यावर्ते पतितोऽस्ति। एकत्र देहस्य अनेके रोगाः, अन्यत्र मनसः चिन्ताः। कार्यवैफल्येन नैराश्यम् भवति। जीवनशैल्याः परिवर्तन विना मार्गान्तरं नास्ति। व्यायामाभावात् शरीरस्य स्थौल्यं वर्धते तारुण्यावस्थायाम् च मुधमेहः जायते।
एतदेव व्याधिः उच्यते। आधिव्याधिभ्यां पीडितो मानवः निद्रां न लभते। मानवः अतृप्त्या सदा खिद्यते। अतः यथाशक्ति शारीरिक श्रमः कर्त्तव्यः। यत्र पद्भ्यां गन्तु शक्यते तत्र वाहनेन न गन्तव्यम। अलं श्रमचिन्त्या।
इदं शरीरं पोषितमपि पालितमपि च शोभते। स्वस्थशरीरेणैव मानवः सोत्साहं गृहस्थजीवनस्य सर्वविधानां समस्यानाम् समाधाने कुशलः भवति। सः आध्यात्मिकज्ञानमपि लभते। उक्तञ्च ‘शरीरमाद्यं खलु धर्मसाधनम्’।
(अ) एकपदेन उत्तरत। (केवलं प्रश्नद्वयम्) (1 × 2 = 2)
(i) कस्य अभावात् शरीरस्य स्थौल्यं वर्धते?
(ii) मानवः कया सदा खिद्यते?
(ii) वर्तमानकाले कः समस्यावर्ते पतितोऽस्ति?
उत्तर
(i) व्यायामस्य
(ii) अतृप्त्या
(iii) मानवः
(आ) पूर्णवाक्येन उत्तरत। (केवल प्रश्नद्वयम्) (1 × 2 = 2)
(i) कार्यवैफल्येन किं भवति?
(ii) स्वस्थशरीरेणैव मानवः कासां समाधाने कुशलः भवति?
(iii) मानवैः यथाशक्ति कः कर्तव्यः?
उत्तर
(i) कार्यवैफल्येन नैराश्यं भवति।
(ii) स्वस्थ शरीरेणैव मानवः सोत्साहं गृहस्थजीवनस्य सर्वविधानां समस्यानां समाधाने कुशलः भवति।
(iii) मानवैः यथाशक्ति शारीरिक श्रमः कर्त्तव्यः।
(इ) अस्य अनुच्छेदस्य कृते उपयुक्तं शीर्षकं संस्कृतेन लिखत। (1 × 2 = 2)
उत्तर
जीवनशैली
(ई) यथानिर्देशम् उत्तरत्-(केवलं प्रश्नत्रयम्) (2 × 2 = 4)
(i) ‘खिद्यते’ इत्यस्याः क्रियायाः कर्तृपदं किम्?
(क) सन्तोषः
(ख) मानवः
(ग) मधुमेहः
उत्तर
(ख) मानवः
(ii) ‘पीडितः मानव’ अनयोः पदयोः विशेषणं किम्?
(क) पीडितः
(ख) मानवः
(ग) नरः
उत्तर
(क) पीडितः
(iii) देहस्य’ इत्यस्य पदस्य कः पर्यायः अत्र आगतः?
(क) मानवस्य
(ख) दुःखस्य
(ग) शरीरस्य
उत्तर
(ग) शरीरस्य
(iv) अनुच्छेदे ‘अन्तिमम्’ पदस्य क : विपर्ययः आगतः?
(क) अलम्
(ख) आद्यम्
(ग) इदम्
उत्तर
(ख) आद्यम्
रचनात्मकं कार्यम् (अङ्काः 15)
प्रश्न 12.
पितरं प्रति लिखिते पत्र रिक्तस्थानानि पूरयित्वा पत्रं च पुनः उत्तरपुस्तिकायां लिखतु। ( 1/2 x 10 = 5)
(i) ……….
दिनाङ्कः ………..
पूज्याः (ii) ………..
सादरं प्रणामाः। अत्र कुशलं तत्रास्तु। इदं विज्ञाय भवान् अतिप्रसन्नः भविष्यति यद् गतदिवसे अन्तर्विद्यालयीयभाषणप्रतिस्पर्धायां मया (iii) …………….. स्थानं लब्धम्। क्रीडादिवसे (iv) ……….. अहमेव प्रथमः आसम्। अस्मिन् वर्षे वार्षिकोत्सवे अहं नाट्याभिनयं (v) ………….”। अयं वार्षिकोत्सवः आगामि-सोमवासरे (vi) ……………………”। विद्यालस्य पक्षतः ह्य एव (vii) ………. प्रेषितम्। अहमपि (viii) … सूचयामि, भवान् अवश्यम् आगच्छतु। मम (ix) …………….. भविष्यति। भवतः आज्ञाकारी पुत्रः, (x) …………..
मञ्जूषा – निमन्त्रणपत्रम्, धावनप्रतियोगितायाम्, पीयूषः, उत्साहवर्धनम्, भवन्तम्, प्रयागराजतः, प्रथमम्, करिष्यामि, पितृमहोदयाः, आयोजयिष्यते।
उत्तर
(i) प्रयागराजतः
(ii) पितृमहोदयाः
(iii) प्रथमम्
(iv) धावनप्रतियोगितायाम्
(v) करिष्यामि
(vi) आयोजयिष्यते
(vii) निमन्त्रणपत्रम्
(viii) भवन्तम्
(ix) उत्साहवर्धनम्
(x) पीयूषः
प्रश्न 13.
प्रदत्तं चित्रं दृष्ट्वा मञ्जूषायां प्रदत्तशब्दानां सहायतया पञ्च वाक्यानि संस्कृतेन लिखत- (1 x 5 = 5)

मञ्जूषा- पति, पत्नी, बालिका, वृक्षा, बहवः, जनाः, परस्परं, वार्तालापं, कुर्वन्ति, लिखति, पत्राणि, वृद्धजनाः, अपि, सन्ति, स्त्रियः पुरुषाः।
उत्तर
(i) इदम् एकं विमानस्थानकस्य चित्रम् अस्ति।
(ii) चित्रे बहवः वृक्षाः सन्ति। वृक्षे पत्राणि अपि सन्ति।
(iii) तत्र पति पत्नी अन्ये जनाः च परस्परं वार्तालापं कुर्वन्ति।
(iv) बालिकाः वृद्धजनाः च सन्ति।
(v) स्त्रियः पत्राणि लिखन्ति।
अथवा
मञ्जूषाप्रदत्तशब्दानां साहाय्येन निम्नलिखितं विषयम् अधिकृत्य पञ्चभिः संस्कृतवाक्यैः एकम् अनुच्छेद लिखत (1 x 5 = 5)
‘पुस्तकमेलकम्’
मञ्जूषा- चित्राणि, पुस्तकानि, मित्राणि, लोकार्पणम्, अभवत्, अतीवप्रसन्ना, ध्वनिमुद्रिकाः, संगणकम्, अन्तःप्रवेशः,
विविधं साहित्यम्, विभिन्नानि, प्रकोष्ठानि, संस्कृत-पुस्तकानि, जनाः, पश्यन्ति।
उत्तर
(i) पुस्तकमेलकं विस्तृते क्षेत्रे आयोजितम् अस्ति।
(ii) तत्र पुस्तकमेलकस्य अन्तः प्रवेशः अतीवप्रसनदायकम् अस्ति।
(iii) पुस्तकमेलके अनेकानि चित्राणि, पुस्तकानि, संगणकं च आसन्।
(iv) तत्र अनेकानि संस्कृत-पुस्तकानि च आसन्।
(v) तत्र विविधं साहित्यम् आसीत् येषां लोकार्पणं भवति स्म।
प्रश्न 14.
अधोलिखितानि वाक्यानि संस्कृतभाषया अनूद्य लिखत-(केवलं वाक्यपञ्चकम् ) (1 x 5 = 5)
(i) लता नाचती है। (Lata dances)
(ii) तुम सब गेंद से खेलते हो। (All of you play by ball)
(iii) कल मोहन बाजार जायेगा। (Mohan will go to the market tomorrow)
(iv) कल रमेश कहाँ था? (Where was Ramesh yesterday?)
(v) रमा सीता के साथ पढ़े! (Rama study with Sita)
(vi) वृक्ष से पत्ते गिरते हैं। (Leaves fall down from the three)
(vii) क्या मैं पढूँ? (May I read?)
उत्तर
(i) लता नृत्यति।
(ii) यूयं कन्दुकेन क्रीडथ।
(iii) श्वः मोहनः आपणं गमिष्यति।
(iv) ह्यः रमेशः कुत्र आसीत्?
(v) रमा सीतया सह पठतु/पठेत्।
(vi) वृक्षात् पत्राणि पतन्ति।
(vii) किम् अहं पठानि?
पठितावबोधनम् (अङ्काः 15)
प्रश्न 15.
अधोलिखितं गद्यांश पठित्वा प्रदत्तप्रश्नानाम् उत्तराणि संस्कृतेन लिखत।। (3)
इति श्रुत्वा व्याघ्रमारी काचिदियमिति मत्वा व्याघ्रो भयाकुलचित्तो नष्टः। भयाकुलं व्याघ्रं दृष्ट्वा कश्चित् धूर्तः शृगालः हसन्नाह- “भवान् कुतः भयात् पलायितः? । व्याघ्रः- गच्छ, गच्छ जम्बुक! त्वमपि किञ्चिद् गूढप्रदेशम्। यतो व्याघ्रमारीति या शास्त्रे श्रूयते तयाहं हन्तुमारब्धा परं गृहीतकरजीवितो नष्टः शीघ्रं तदग्रतः। शृगालः- व्याघ्रः त्वया महत्कौतुहलम् आवेदितं यन्मानुषादपि बिभेषि? व्याघ्रः- प्रत्यक्षमेव मया सात्मपुत्रावेकैशो मामत्तुं कलहायमानौ चपेटया प्रहरन्ती दृष्टा।
(अ) एकपदेन उत्तरत। (केवलं प्रश्नद्वयम्) (1/2 × 2 = 1)
(i) शृगालस्य अपरं नाम किम् । ‘शृगालः’ इति शब्दाय किम् अन्यः शब्दः प्रयुक्तः?
(ii) कीदृशः व्याघ्रः नष्ट:?
(iii) शृगालः किम् कुर्वन् अवदत्?
उत्तर
(i) जम्बुकः
(i) भयाकुलचित्तः
(iii) हसन्
(आ) पूर्णवाक्येन उत्तरत। (केवल प्रश्नद्वयम्) (1 × 2 = 2)
(i) व्याघ्रः भयाकुलचित्तः किमर्थम् अधावत्?
(ii) व्याघ्रः जम्बुकम् प्रति किम् कथयति?
(iii) प्रत्यक्षमेव सिंहेन किम् दृष्टा?
उत्तर
(i) इयं काचित् व्याघ्रमारी इति मत्वा व्याघ्रः भयाकुलचित्तः अधावत्।
(ii) गच्छ गच्छ जम्बुक! त्वमपि किञ्चित् गूढप्रदेशम् इति व्याघ्रः जम्बुकं प्रति अकथयत्।
(iii) प्रत्यक्षमेव व्याघ्रण सात्मपुत्रावेकैकशो तम् अत्तुं कलहायमानौ चपेटया प्रहरन्ती दृष्टा।
प्रश्न 16.
अधोलिखितं पद्यांशं पठित्वा प्रदत्तप्रश्नानाम् उत्तराणि संस्कृतेन लिखत
निमित्तमुद्दिश्य हि यः प्रकुप्यति,
ध्रुवं स तस्यापगमे प्रसीदति।
अकारणद्वेषि मनस्तु यस्य वै,
कथं जनस्तं परितोषयिष्यति।।
(अ) एकपदेन उत्तरत। (केवलं प्रश्नद्वयम्) (1/2 × 2 = 1)
(i) अत्र किम् अकारणं द्वेषि उक्तम्?
(ii) किम् उद्दिश्य यः प्रकुप्यति?
(iii) कस्य अपगमे मनुष्यः प्रसीदति?
उत्तर
(i) मनः
(ii) निमित्तम्
(iii) निमित्तस्य/तस्य
(आ) पूर्णवाक्येन उत्तरत। (केवलं प्रश्नद्वयम्) (1 × 2 = 2)
(i) मनुष्यः कदा ध्रुवं प्रसीदति?
(ii) कं जनः कथमपि न परितोषयिष्यति?
(iii) निमात्तापगमे जनः किं करोति?
उत्तर
(i) मनुष्यः निमित्तस्य अपगमे ध्रुवं प्रसीदति।
(ii) कारणद्वेषि मनस्तु कथमपि जन: न परितोषयिष्यति।
(iii) निमित्तापगमे जनः प्रसीदति
प्रश्न 17.
अधोलिखितं नाट्यांशं पठित्वा प्रदत्तानां प्रश्नानाम् उत्तराणि संस्कृतेन लिखत। (3)
व्याघ्र चित्रकौ:- अरे किं वनराजपदाय सुपात्रं चीयते?
एतदर्थं तु आवामेव योग्यौ। यस्य कस्यापि चयनं कुर्वन्तु सर्वसम्मत्या।
सिंहः- तूष्णीं भव भोः। युवामपि मत्सदृशौ भक्षको न तु रक्षको। एवे वन्यजीवाः भक्षकं रक्षकपदयोग्यं न मन्यते अत एव विचारविमर्शः प्रचलित।
बाक : – सर्वथा सम्यगुक्तम् सिंहमहोदयेन। वस्तुतः एव सिंहेन बहुकालपर्यन्तं शासनं कृतम् परमधुना तु कोऽपि पक्षी एव राजेति निश्चंतव्यम् अत्र तु संशीतिलेशस्यापि अवकाशः एव नास्ति।
उत्तर
(अ) एकपदेन उत्तरत। (केवल प्रश्नद्वयम्) (1/2 x 2 = 1)
(i) कस्मै पदाय सुपात्रं चीयते?
(ii) आवाम् एव योग्यौ इति को वदत:?
(iii) “युवाम् अपि मत्सदृशौ भक्षको।” अत्र युवाम् पदं काभ्याम् प्रयुक्तम्?
उत्तर
(i) वनराज
(i) व्याघ्रचित्रको
(iii) व्याघ्रचित्रकाभ्याम्
(आ) पूर्णवाक्य में उत्तरत। (केवल प्रश्नद्वयम्) (1 x 2 = 2)
(i) सिंहः किम् कथयति?
(ii) बकः सिंह किम् अकथयत्?
(iii) व्याघ्रचित्रकौ वनराजपदाय किम् अकथयताम्?
उत्तर
(i) सिंहः कथयति-तूष्णीं भव भोः! युवामपि मत्सदृशौ भक्षकौ न तु रक्षको।
(ii) बक: सिंह कथयति-सवर्था सम्यगुक्तं सिंह महोदयेन। बहुकालपर्यन्तं सिंहेन शासनं कृतम् अधुना तु कोऽपि पक्षी एव राजा भवेत्।
(iii) व्याघ्रचित्रकौ अकथयताम्-अरे किं वनराजपदाय सुपात्रं चीयते? एतदर्थं तु आवामेव योग्यौ।
प्रश्न 18.
मञ्जूषातः समुचितपदानि चित्वा अधोलिखित-श्लोकस्य अन्वयं पूरयत (1/2 x 4 = 2)
1. श्लोक- उदीरितोऽर्थः पशुनापि गृह्यते,
हयाश्च नागाश्च वहन्ति बोधिताः।
अनुक्तमप्यूहति पण्डितो जनः,
परेङ्गितज्ञानफला हि बुद्धयः।।
अन्वय- (i) ……. …… अपि उदीरितः अर्थः …. … गृह्यते, बोधिताः हयाः च (ii) …………. “च (भारम्) वहन्ति। (iii) …….. जनः अनुक्तम् अपि ऊहति। (iv)…………… परेङ्गितज्ञानफलाः हि (भवन्ति)।
अथवा
II . श्लोक- क्रोधो हि शत्रुः प्रथमो नराणां,
देहस्थितो देहविनाशनाय।।
यथास्थितः काष्ठगतो हि वह्निः,
स एव वह्निर्दहते शरीरम्।।
अन्वय- क्रोधः हि (i) ……………. देहविनाशाय देहस्थितः (ii) ……………. शत्रुः (अस्ति)। यथा (iii) ………… स्थितः हि वहिन (भवति) सः वह्निः काष्ठम् एव दहते तथा (iv) …. …… शरीरम् दहते। मञ्जूषा- प्रथमः, नागाः, बुद्धयः, क्रोधः, पशुना, पण्डितः, नराणां, काष्ठगतः
उत्तर
I. (i) पशुना
(ii) नागाः
(iii) पण्डितः
(iv) बुद्धयः
II. (i) नराणां
(ii) प्रथमः
(iii) काष्ठगतः
(iv) क्रोधः
अथवा
मञ्जूषायाः साहाय्येन श्लोकस्यभावार्थे रिक्तस्थानानि पूरयित्वा पुनः लिखत। श्लोक- वाक्पटुधैर्यवान् मंत्री सभायामप्यकातरः।
स केनापि प्रकारेण परैर्न परिभूयते।। भावार्थ- य मन्त्री
(i) ……………. सम्भाषणे चतुरः, धैर्ययुक्तः,
(ii) …………. च भवति, सः मन्त्री
(iii) …………………. कथमपि
(iv)…………………. न शक्यते। मञ्जूषा- तिरस्कर्तुम्, निर्भीकः, सभायाम्, विरोधिभिः
उत्तर
(i) सभायाम्
(ii) निर्भीकः
(iii) विरोधिभिः
(iv) तिरस्कर्तुम्
प्रश्न 19.
अधोलिखित-कथांशं समुचितक्रमेण लिखत।। ( 1/2 x 8 =4)
(i) तत्र निहितामेकां मञ्जूषाम् आदाय पलायितः।
(ii) चौरः एव उच्चैः क्रोशितुमाराभत “चौरोऽयं चौरोऽयम्” इति।
(iii) चौरस्य पादध्वनिना प्रबुद्धोऽतिथि: चौरशङ्कया तमन्वधावत् अगृह्णाच्च, परं विचित्रमघटत।
(iv) तस्योमेव रात्रौ कश्चन चौर: गृहाभ्यन्तरं प्रविष्टः।
(v) विचित्रा दैवगतिः
(vi) तस्य तारस्वरेण प्रबुद्धः ग्रामवासिनः स्वगृहाद् निष्क्रम्य तत्रागच्छन् वराकमतिथिमेव च चौरं मत्वाऽभर्त्सयन्।
(vii) यद्यपि ग्रामस्य आरक्षी एव चौर आसीत्।
(vii) तत्क्षणमेव रक्षापुरुषः तम् अतिथिं चौरोऽयम् इति प्रख्याप्य कारागृहे प्राक्षिपत्।
उत्तर
(i) विचित्रा दैवगतिः।
(ii) तस्यामेव रात्रौ
(iii) तत्र निहितामेकां
(iv) चौरस्य पादध्वनिना …..
(v) चौरः एव उच्चैः ………….
(vi) तस्य तारस्वरेण
(vii) यद्यपि ग्रामस्य आरक्षी …
(viii) तत्क्षणमेव रक्षापुरुषः
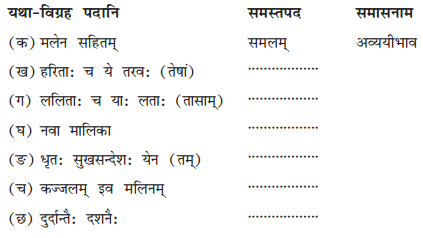
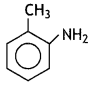
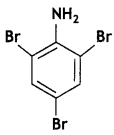
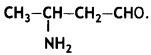
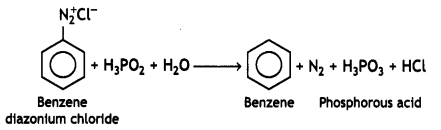
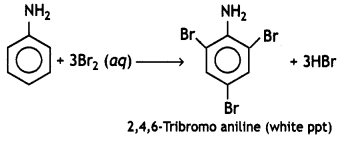
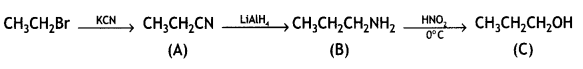
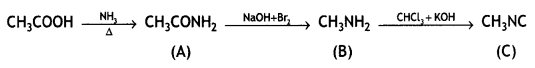
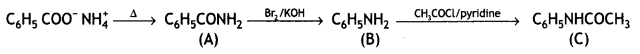
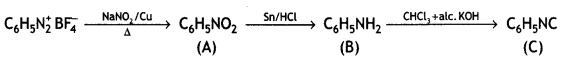
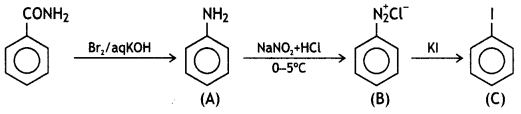
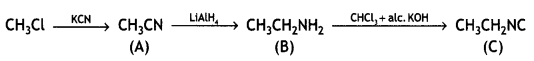
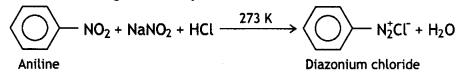
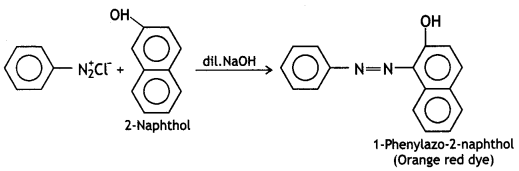
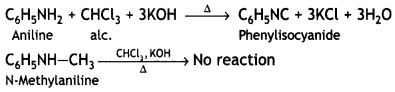
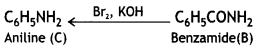
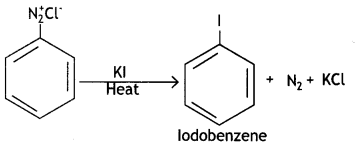
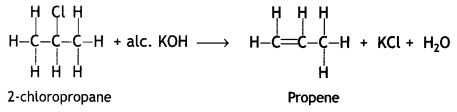
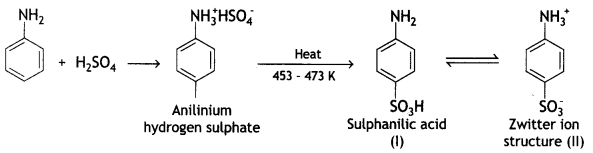
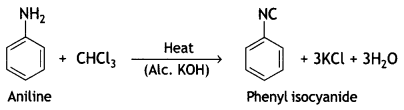
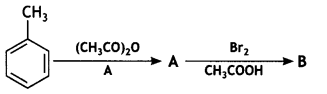
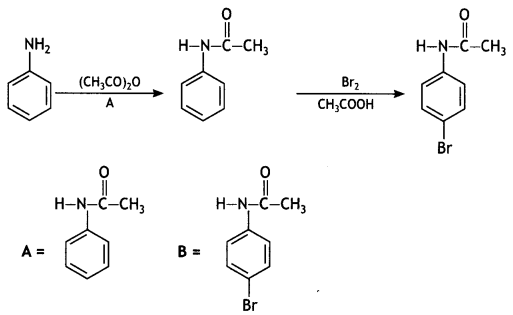

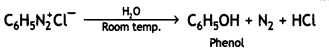
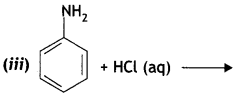
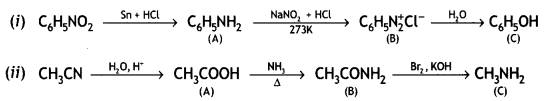
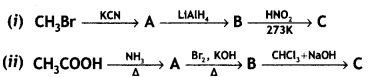
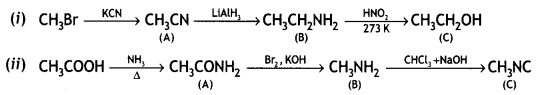
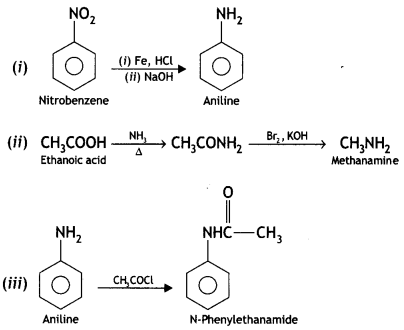
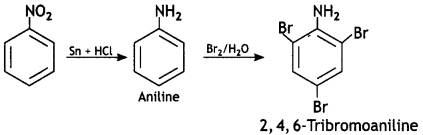
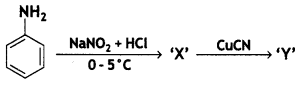
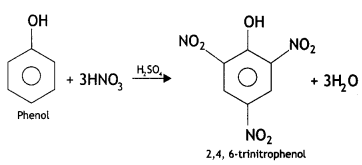
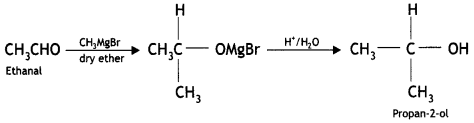
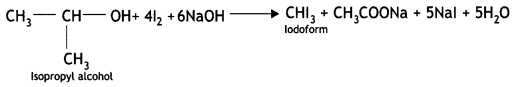
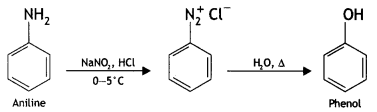
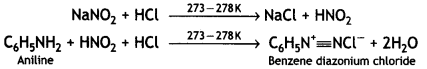
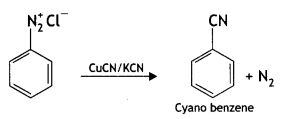
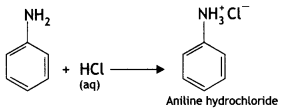
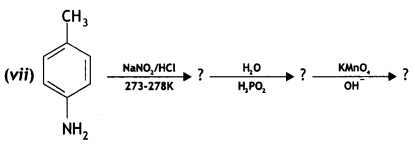
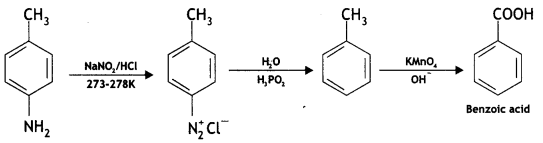
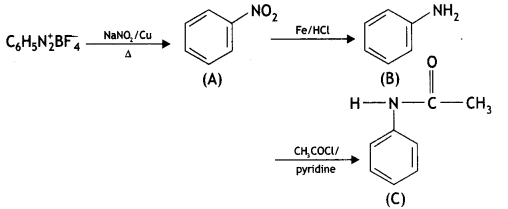
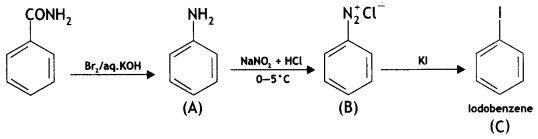
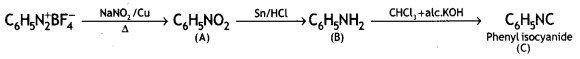
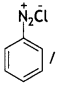 benzene diazonium ch(orlde,
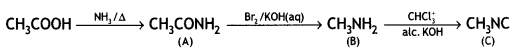
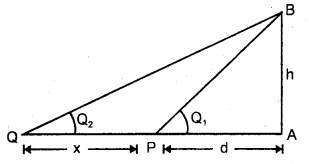
benzene diazonium ch(orlde,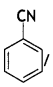 Cyanobenzene
Cyanobenzene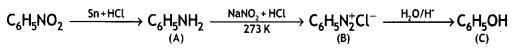
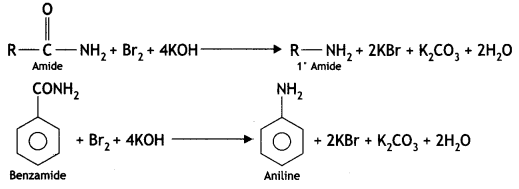
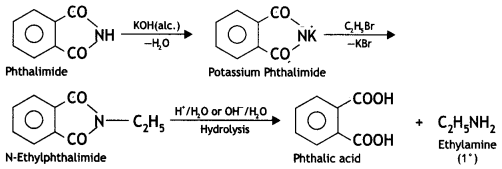
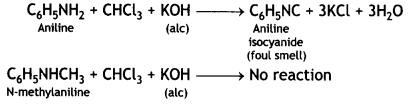
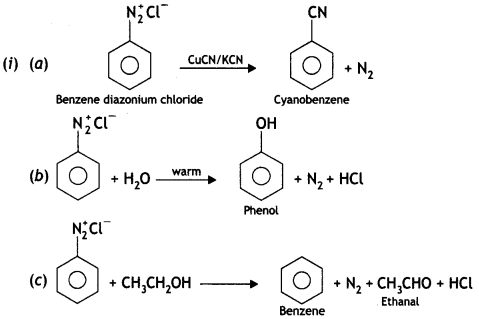
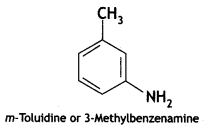
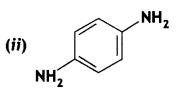
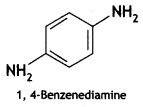
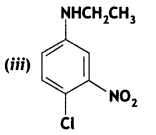
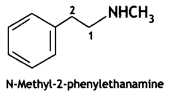
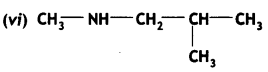
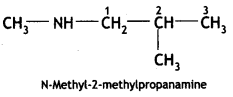
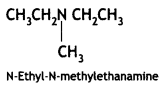


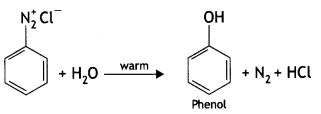
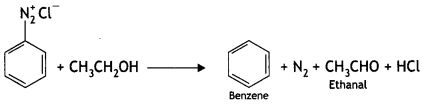
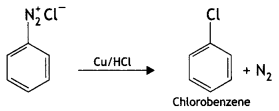
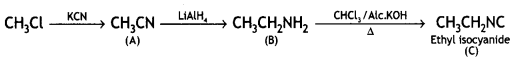
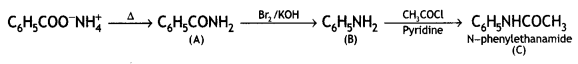
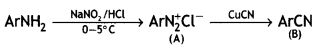
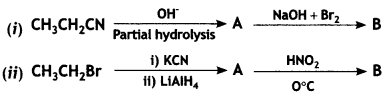
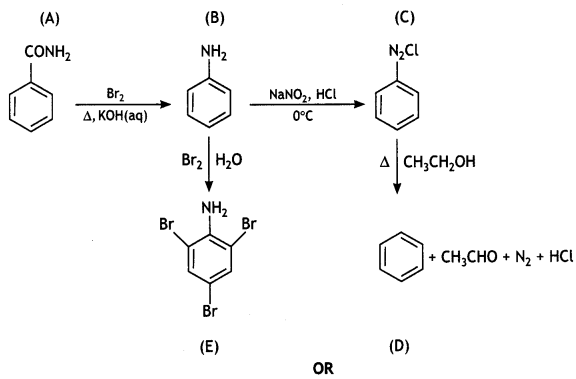

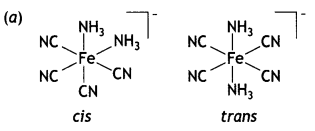
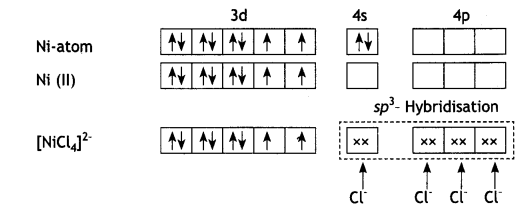
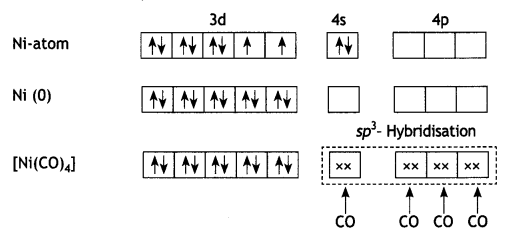
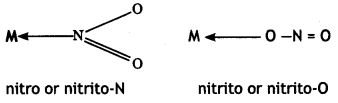
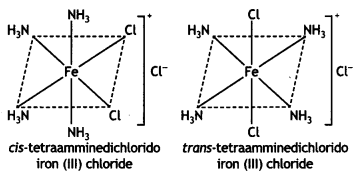
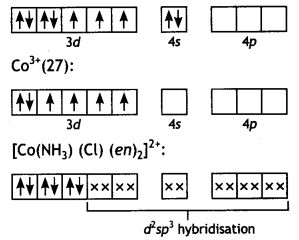
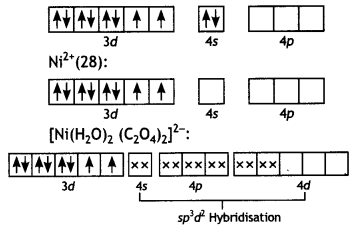
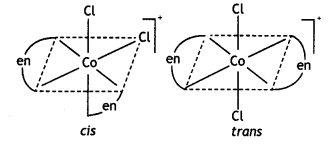

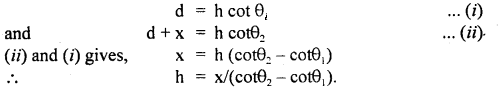
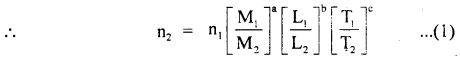

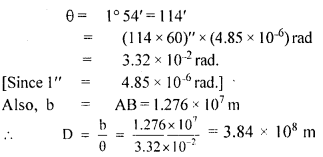
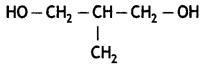
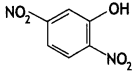
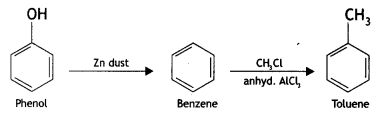
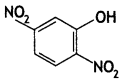 2, 5- Dinitrophenol
2, 5- Dinitrophenol