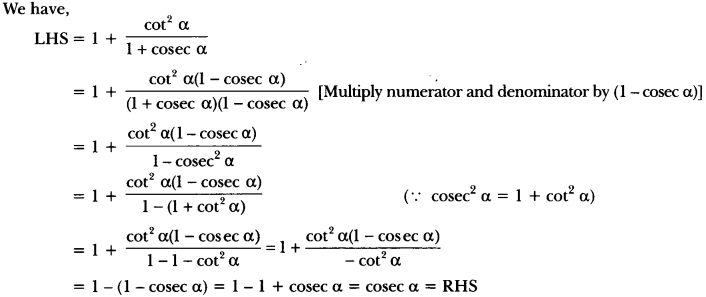
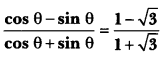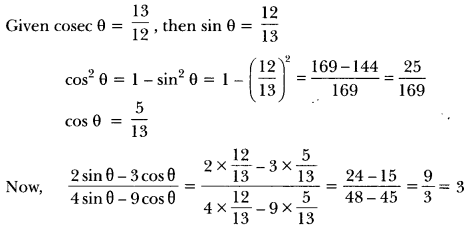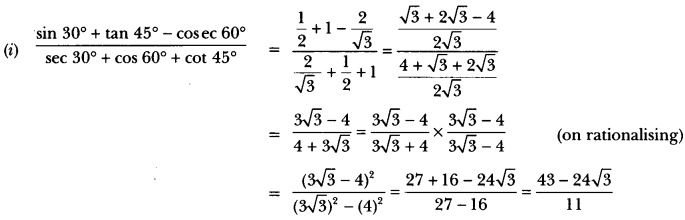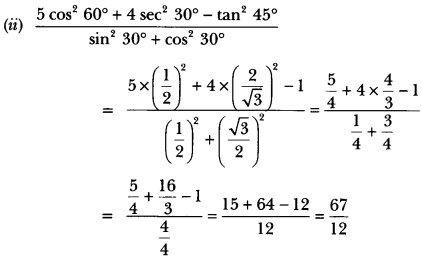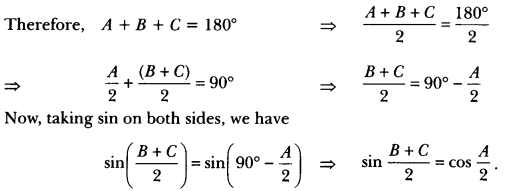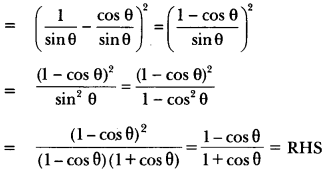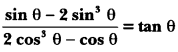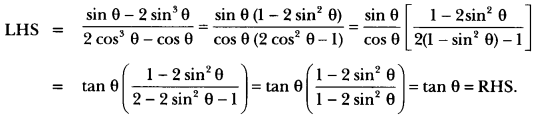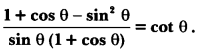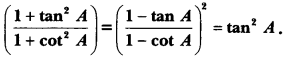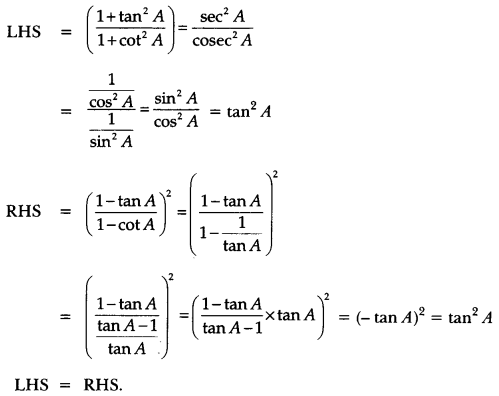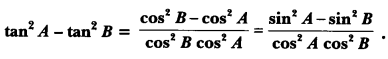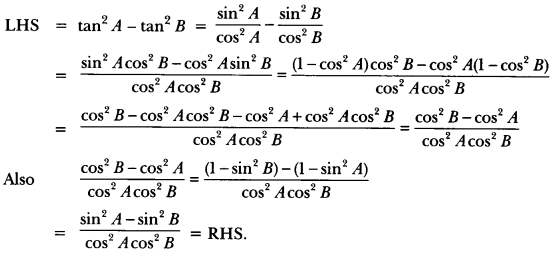In this page, we are providing Control and Coordination Class 10 Extra Questions and Answers Science Chapter 7 pdf download. NCERT Extra Questions for Class 10 Science Chapter 7 Control and Coordination with Answers will help to score more marks in your CBSE Board Exams. https://ncertmcq.com/extra-questions-for-class-10-science/
Class 10 Science Chapter 7 Extra Questions and Answers Control and Coordination
Extra Questions for Class 10 Science Chapter 7 Control and Coordination with Answers Solutions
Extra Questions for Class 10 Science Chapter 7 Very Short Answer Type
Question 1.
Define ‘reflex action’. [CBSE 2009]
Answer:
It is an automatic, spontaneous and an immediate involuntary response to a stimulus controlled usually by the spinal cord. e.g. knee jerk movement.
Question 2.
Name the largest cell in the human body. [CBSE 2003]
Answer:
Nerve cell (Neuron).
Question 3.
Give an example of plant hormone that promotes growth. [CBSE 2008]
Answer:
Auxin
Question 4.
What are plant hormones? [CBSE 2008]
Answer:
The chemical substances produced in plants which help in the growth and development of plant, its tissues and other plants.
Question 5.
Name the part of neuron [CBSE 2008]
(а) where information is acquired.
(b) through which information travels an electrical impulse.
Answer:
(a) Dendrite
(b) Axon
Question 6.
Name two tissues that provide control and coordination in multicellular animals. [CBSE 2009]
Answer:
Muscular tissues and nervous tissue.
Question 7.
Name the hormone, the secretion of which is responsible for dramatic changes in appearance in girls and boys when they approach 10-12 years of age. [CBSE 2008]
Answer:
Testosterone released from testes in males, estrogen released from ovaries in females.
Question 8.
Name the hormone that helps in regulating level of sugar in our blood. Name the glands that secretes it. [CBSE 2010]
Answer:
Insulin hormone secreted by pancreas.
Question 9.
Name a plant hormone that inhibits growth. Write its one more function. [CBSE 2014]
Answer:
Abscisic acid inhibits growth in plants. It also causes closure of stomata during water stress.
Question 10.
A potted plant is made to lie horizontally on the ground. Which part of the plant will show:
(i) Positive geotropism
(ii) Negative geotropism [CBSE 2010]
Answer:
(i) Roots will shows positive geotropism.
(ii) Shoots will show negative geotropism.
Question 11.
Name a plant hormone which promotes growth in plants. [CBSE 2008]
Answer:
Auxin is a plant hormone that promotes growth and cell elongation in plants.
Question 12.
State one function each of pons and cerebellum.
Answer:
Pons: Regulates rate of respiration
Cerebellum: Maintains equilibrium of the body during walking, jumping, etc.
Question 13.
Name a gaseous plant hormone. Give its role.
Answer:
Ethylene is a gaseous hormone. It regulates fruit ripening.
Question 14.
How many spinal and cranial nerves are present in human body?
Answer:
Spinal nerves = 31 pairs
Cranial nerves = 12 pairs
Question 15.
What are meninges?
Answer:
The three membranes which cover the brain to protect it are called meninges.
Extra Questions for Class 10 Science Chapter 7 Short Answer Type I
Question 1.
How do we detect smell of hot spicy food from a distance?
Answer:
We have olfactory receptors in our nose which detect the smell of hot spicy food.
This information is transmitted by nerve impulse to olfactory lobes of forebrain which interpret the information.
Question 2.
Why do tendrils coil around hard objects or support?
Answer:
The tendrils coil around hard objects or support due to a stimulus of touch (thigmotropism) which causes less growth on the side in contact with support than the side which is away from it. This unequal growth of two sides of tendril makes it coil around the support.
Question 3.
Name the hormones reponsible for regulation.
(i) Metabolism of carbohydrates, fats and proteins
(ii) Balance of calcium and phosphate
(iii) Blood pressure
(iv) Water and electrolyte balance. [CBSE 2007]
Answer:
(i) Thyroxin
(ii) Parathyroid hormone
(iii) Adrenaline
(iv) Vasopressin
Question 4.
What is an association neuron?
Answer:
Association neurons are present in cortex part of spinal cord between the sensory neuron and motor neuron. It helps to interconnect the signals between the sensory neuron and motor neuron by forming synapse with axon of sensory neuron and dendrite of motor neuron.
Question 5.
How are the brain and spinal cord protected from mechanical shock?
Answer:
Brain is present in a bony box called cranium (skull), spinal cord is protected by vertebral column. The cerebrospinal fluid present around the brain and spinal cord protect it from mechanical shock.
Question 6.
Which functions are regulated by the forebrain?
Answer:
The thinking part of the brain is forebrain which controls
- Movement of voluntary muscles.
- Hearing, smell, sight, hunger, thirst, pain, etc. by its association areas.
- It also stores information and controls intelligence.
Question 7.
Explain how a squirrel responds a dangerous situation with help of its hormonal system.
Answer:
When a squirrel perceives a dangerous situation, adrenaline hormone is released in its blood which increases its heart beat and blood supply to tissues. This provides energy to its cells and tissues at a faster rate and enables it to run away from emergency situation.
Question 8.
How are sensory neurons different from motor neurons?
Answer:
Sensory neuorons take information from receptors and transmit the impulses towards central nervous system. Motor neurons carry message from control nervous system to the muscle, gland or an organ to enable it to respond.
Question 9.
How are receptors different from effectors?
Answer:
Receptors are cells, tissues or organs which receive the information in form of stimulus. For example, photoreception, gustatory receptors, etc. Effectors are muscles, glands tissues or cells which respond according to the information received through motor neuron from the central nervous system.
Question 10.
Define peripheral nervous system. What are its components?
Answer:
The nerves that directly arise from the central nervous system and contact different parts of our body to help their involuntary controls. Spinal nerves and cranial nerves are components of peripheral nervous system.
Question 11.
Lable the parts (a), (b), (c) and (d) and show the direction of flow of electrical signals in the given figure.
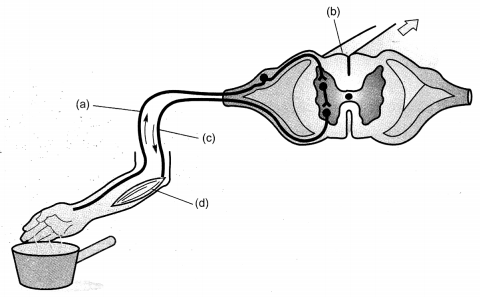
Answer:
(a) Sensory neuron
(b) Spinal cord (part of CNS)
(c) Motor neuron
(d) Effector (Muscle)
Question 12.
Name the plant hormones responsible for the following:
(a) elongation of cells
(b) growth of stem
(c) promotion of cell division
(d) falling of senescent leaves
Answer:
(a) Auxin
(b) Gibberellin
(c) Cytokinin
(d) Abscissic acid
Question 13.
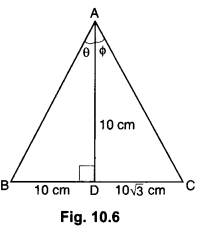
Label the endocrine glands in figure given below.
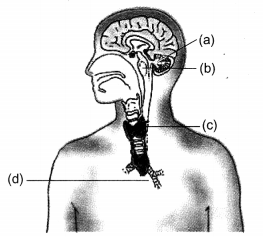
Answer:
(a) Pineal Gland
(b) Pituitary Gland
(c) Thyroid
(d) Thymus
Question 14.
Label the parts of a neuron in figure given below.
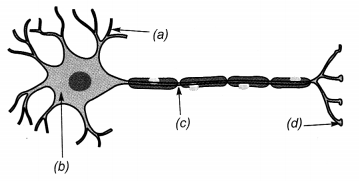
Answer:
(a) Dendrite
(b) Cell Body
(c) Axon
(d) Nerve ending
Question 15.
Match the terms of Column (A) with those of Column (B)

[NCERT Exemplar]
Answer:
(a) (iii)
(b) (iv)
(c) (i)
(d) (ii)
Extra Questions for Class 10 Science Chapter 7 Short Answer Type II
Question 1.
What are nastic and curvature movements? Give one example of each. [CBSE 2010]
Answer:
The non-directional responses to stimuli are called nastic movements e.g., drooping of leaves of touch-me-not plant. Curvature movement are the movement of plant parts towards or away from stimulus e.g., bending of shoot towards light.
Question 2.
How does feedback mechanism regulate the hormone action? Explain with the help of an example.
Answer:
The presence or absence of a particular hormone can regulate its further formation with the help of a regulatory mechanism called feedback mechanism.
Example: Hypothalamus regulates thyroxin levels in blood by secreting thyroid stimulating hormone (TSH). If the thyroxine levels increases then hypothalamus stop secreting TSH in order to reduce the production of thyroxine from thyroid gland.
Low levels of thyroxin in blood again switches on the release of TSH from hypothalamus to increase levels of thyroxin in blood.
Question 3.
What are the different types of neurons and their functions in the human body?
Answer:
There are mainly three types of neurons:
- Sensory neuron: They transmit information from receptors towards the central nervous system.
- Motor neuron: They transmit information from the central nervous system to effectors like muscles or glands.
- Relay neuron: It serves as a link between the sensory and the motor neurons in the brain or spinal cord.
Question 4.
What are the limitations to the use of electrical impulse?
Answer:
The limitations to electrical impulse are
- Only those cells used that are connected by nervous tissue, while other tissues do not receive the information directly.
- It takes some times to reset the mechanism of generation of new electrical impulse once an electrical impulse had been generated.
Question 5.
Depict the mechanism of Nervous tissue action.
Answer:
The mechanism of nervous tissue action is:
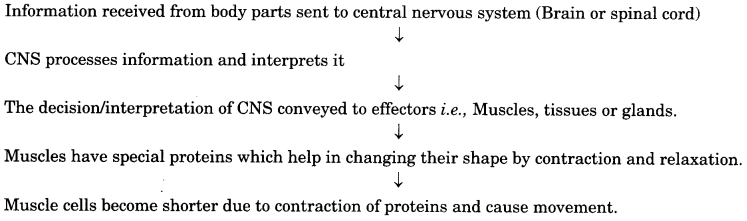
Question 6.
What is a tropic movement? Explain with an example. [NCERT Exemplar]
Answer:
The directional movements caused in plants due to an external stimuli are called tropic movements.
Example: During phototropism, the shoot bends towards light and show positive phototropism while the roots bend away from light to show negative phototropism.
Question 7.
What will happen if intake of iodine in our diet is low? [NCERT Exemplar]
Answer:
Iodine helps in the synthesis of thyroxin hormone from thyroid gland. Thyroxin hormone is necessary for carbohydrate, proteins and fat metabolism.
Deficiency caused due to low level of iodine in diet might result is a disease called goitre in the person.
Question 8.
Answer the following:
(a) Which hormone is responsible for the changes noticed in females at puberty?
(b) Dwarfism results due to deficiency of which hormone?
(c) Blood sugar level rises due to deficiency of which hormone?
(d) Iodine is necessary for the synthesis of which hormone? [NCERT Exemplar]
Answer:
(a) Oestrogen
(b) Growth hormone
(c) Insulin
(d) Thyroxin
Question 9.
Answer the following:
(a) Name the endocrine gland associated with brain.
(b) Which gland secretes digestive enzymes as well as hormones?
(c) Name the endocrine gland associated with kidneys.
(d) Which endocrine gland is present in males but not in females? [NCERT Exemplar]
Answer:
(a) Pituitary
(b) Pancreas
(c) Adrenal gland
(d) Testes
Extra Questions for Class 10 Science Chapter 7 Long Answer Type
Question 1.
Differentiate between tropic and nastic movements in plants. [CBSE 2006]
Answer:
Tropic Movements:
- There is directional growth of a plant or part of a plant in response to an external stimulus i.e., direction of stimulus controls direction of growth.
- The effect is more or less permanent.
- It is easily observed in stems and roots of plants.
- It occurs due to unequal growth on the two sides of a stem or root.
- For example, bending of root towards gravity and bending of shoot towards light.
Nastic Movements:
- The growth or movement is independent of the direction of stimulus.
- The effect is temporary and reversible.
- It occurs in specialised parts and organs of plants like leaves and petals of flowers.
- It usually involves alterations in cell volumes.
- For example, folding on leaflets of touch-me-not plant on touching them.
Question 2.
Mention one function for each of these hormones:
(a) Thyroxin
(b) Insulin
(c) Adrenaline
(d) Growth hormone
(e) Testosterone
Answer:
(a) Thyroxin: It regulates carbohydrates, fat and protein metabolism.
(b) Insulin: It regulates blood sugar level.
(c) Adrenaline: It increases heart beat rate and supply of blood to various organs.
(d) Growth hormones: It regulates growth and development of an organism.
(e) Testosterone: It controls the bodily features, secondary sexual characters in males during puberty.
Question 3.
How does chemical coordination take place in animals?
Answer:
Hormones secreted by the endocrine glands are directly poured into the blood stream as they are ductless glands. Blood carries these hormones to specific target tissue or organ where they act and trigger a particular biochemical or physiological activity in response to the stimulus received.
Question 4.
Why is the flow of signals in a synapse from axonal end of one neuron to dendritic end of another neuron but not the reverse? [NCERT Exemplar]
Answer:
The information received by the dendrites of neurons present at receptors is transferred in form of electrical impulse to the cell body, axon and the nerve endings at the ends of axon. At the axonal ends, chemicals are released between junction of two neurons called synapse. The chemical diffuses towards the dendrite of the next neuron where it generates an electrical impulse again. So, the electrical signals change to chemical signals and again to electrical signals for the next neuron.
Since the chemicals Eire released at the axonal ends and absent at dendrite end, the signal travels from axonal end to dendritic end of another neuron but not the reverse i.e., the flow of electrical impulse is unidirectional only.
Control and Coordination HOTS Questions With Answers
Question 1.
While most of our actions are coordinated by the brain, still some of our actions need to be coordinated by the spinal cord. Why?
Answer:
Brain is the thinking part which coordinates the various activities and processes in human beings. But, some actions which need to be quick, automatic and involuntary, do not involve any thinking processes.
So, such immediate responses are coordinated by the spinal cord and are called reflex actions.
Question 2.
The movements shown by the leaves of ‘touch me not’ plant on touching are different from the movement shown by seedling. Elaborate.
Answer:
The opening and closing of the leaves of the ‘touch me not plant’ is a result of water change in amount of water in the plant cells, which is not dependent on growth. But, the movement of the seedling is a growth-dependent movement because it will not show any movement if it is prevented from growth.
Question 3.
The level of hormones should be well balanced in human beings in order to maintain the normal functioning of the human body. Explain this statement with two examples.
Answer:
The level of hormones should be balanced in human beings because a deficiency or excess secretion of some hormones can have adverse effects on the human body. For example,
- A deficiency in the secretion of insulin from the pancreas increases the blood sugar level and causes diabetes.
- A deficiency in the secretion of growth hormone causes dwarfism whereas it excess secretion causes gigantism.
Question 4.
Chemical coordination plays a vital role in the activities of plants. Elaborate.
Answer:
Coordination in human beings is carried out both by the nervous as well as the hormonal system. But, coordination in plants is dependent on the chemicals called as hormones. The hormone auxin and gibberellins help in the growth of the stem. Cytokinins help in cell division. Abscisic acid inhibits growth. Auxin is also involved in the bending of plants towards light.
Question 5.
The response of the body due to reflex actions is faster than those carried out by secretion of adrenaline in emergency situations. Why?
Answer:
The reflex actions are the result of chemical-electrical impulses which are faster as they move through the nerve cell from one point to another, whereas, hormones are first released in the blood and they have to travel to the target site to bring about the response, which takes more time than reflex actions.
Extra Questions for Class 10 Science Chapter 7 Value Based Questions
Question 1.
Villagers living in a hilly area were facing a problem that a lot of children in the village were developing swollen necks. The priest of the village told that it was due to the acts of the villagers who had annoyed their Goddess and they needed to offer sacrifice for getting rid of this problem. Dr. Kamal visited the village and properly listened to the problem of the villagers and advised them to use iodised salt instead of normal salt in their food. Villagers agreed to Dr. Kamal’s advice and within a few months the problem was overcome to a large extent.
(a) What was the cause of the swollen neck of children in the village?
(b) What values are shown by Dr. Kamal?
(c) Why was use of iodised salt advised to villagers by Dr. Kamal?
Answer:
(a) The swollen neck is a disease called goitre. Goitre occurs due to deficiency of thyroxine hormone.
(b) Scientific aptitude, patience, problem solving ability and helpful nature.
(c) The use of iodised salt was advised because Iodine helps in production of thyroxine hormone which helps to prevent goitre.
Question 2.
Akhilesh saw a plant in his school garden which closed its leaves on touching. He observed the plant very closely for a long time. In the recess, he saw that some students were trying to pluck the plant from the pot. He went to them and advised them not to pluck the plant as it was their school’s property. He also told them about the importance of the plants and the peculiar behavior of the said plant. In this way, he was able to convince the students to not harm the plant.
(a) Why did the leaves of the plant close on touching?
(b) How is this response of plant different from reflex action in animals?
(c) What values are shown by Akhilesh?
Answer:
(a) The leaves of the sensitive plants like Touch-me-not close on touching as they lose the turgor pressure in cells which causes drooping of its leaves/leaflets.
(b)
Movement in Sensitive Plant:
- The response is produced due to the change in amount of water in the plant cells.
- No nerve or specialised tissue involved in the conduction of information.
Reflex action:
- An automatic, immediate, involuntary response of an organism.
- It is controlled by the spinal cord.
(c) Environment friendly, responsible, leadership, analytical thinking.
Question 3.
Smita’s father was complaining about frequent urination, pain in legs and a frequent weight loss to Smita’s mother and she discussed the things with her daughter when Smita returned from school. Listening to this Smita told her mother that her father should go and visit a doctor immediately. The doctor diagnosed that Smita’s father was having an elevated level of blood glucose. He should take care of his diet and should exercise regularly to maintain his normal glucose level.
On the basis of the text, answer the following questions:
(i) Name the disease he is suffering from and name the hormone whose deficiency causes it.
(ii) Identify the gland that secretes it and mention the function of this hormone.
(iii) Explain how the time and amount of secretion of this hormone is regulated in human system.
(iv) What values were shown by Smita and her father? [CBSE 2012, 2015]
Answer:
(i) Disease-Diabetes, Hormone: Insulin (deficiency of insulin hormone causes diabetes)
(ii) Gland-Pancreas: The blood glucose level is regulated by insulin hormone secreted by the pancreas.
(iii) Cells of pancreas secrete insulin hormone when level of blood glucose level increases in the blood. Insulin regulates the blood glucose level and its secretion gets reduced when blood glucose level falls down.
(iv) Decision making, intelligence, scientific aptitude and caring nature is shown by Smita. Her father had shown an attitude of carelessness towards his health.
Question 4.
Rajesh found an old man lying on the road asking for help to take him to the hospital. He took the old man to the hospital where doctor told him that the old man was suffering from paralysis, and his blood sugar levels were high. The doctor told him that they have given the old man some injections to reduce the blood sugar level and thanked Rajesh for his helpful attitude.
On the basis of above passage, answer the following:
(i) What can be the cause of high blood sugar levels in the old man?
(ii) Which part of old man’s body might have been effected that has caused paralysis?
(iii) What values have been shown by Rajesh?
(iv) Which injection might have been given to old man by doctor to lower the blood sugar levels?
Answer:
(i) Diabetes due to the lower levels of insulin hormone in the body.
(ii) Nervous system associated with brain or spinal cord might have been effected.
(iii) Helpful, care for elderly.
(iv) Injections of insulin might have been given by the doctor to the old man to lower his blood sugar levels.