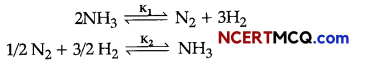Here we are providing Online Education for Mother’s Day Extra Questions and Answers Class 11 English Snapshots, Extra Questions for Class 11 English was designed by subject expert teachers. https://ncertmcq.com/extra-questions-for-class-11-english/
Online Education for Mother’s Day Important Extra Questions and Answers Class 11 English Snapshots
Mother’s Day Extra Questions and Answers Short Answer Type
Mother’s Day Extra Questions Question 1.
Who is Mrs Fitzgerald? What does she advise Mrs Pearson?
Answer:
Mrs Fitzgerald is Mrs Pearson’s neighbour and friend. A fortune teller, who had learnt the art from the East, she tells Mrs Pearson that her fortune could turn either way. With effort and counsel, the situation would swing in her favour. She advised her to assert herself as the boss of the house.
Mother’s Day Class 11 Questions And Answers Question 2.
What was Mrs Pearson’s reaction to Mrs Fitzgerald’s advice?
Answer:
Mrs Pearson said that it would not be easy to put her family members in place as she was very fond of them. She knew that they were thoughtless and selfish but felt, perhaps, they did not mean to be so.
Mother’s Day Class 11 Extra Questions Question 3.
What was Mrs Fitzgerald’s opinion of Mrs Pearson’s attitude?
Answer:
Mrs Fitzgerald said that Mrs Pearson’s family was undoubtedly spoilt. She felt that it was Mrs Pearson’s attitude that did them no good, tending to their needs, taking their orders, and staying at home every night while they went out enjoying themselves.
Mothers Day Questions And Answers Question 4.
What does Mrs Fitzgerald offer to do for her?
Answer:
Mrs Fitzgerald sensed that Mrs Pearson was far too gentle, submissive and generous to tackle her family. Mrs Fitzgerald offered to make them realize the error of their ways not as Mrs Fitzgerald but as Mrs Pearson. She offered to change their bodies and change back again.”
Mother’s Day Class 11 Extra Questions And Answers Question 5.
How did the two women react after their bodies were changed?
Answer:
When Mrs Pearson looked down at herself in Mrs Fitzgerald’s body, she gave a scream of fright. On the other hand, Mrs Fitzgerald is rather pleased and feels that the transition was so neat that she did not even know that she had it in her.
Mother’s Day Extra Questions And Answers Question 6.
What is Doris’s first reaction on seeing her mother? Why?
Answer:
Doris was taken aback to see her mother smoking and playing cards. When Doris asks her what she was doing, she is startled to get her answer—‘whitewashing the ceiling.’ Moreover, her conduct was not nervous and apologetic but cool and incisive.
Mother’s Day Important Questions Question 7.
What did Doris want her mother to do? How did the mother react?
Answer:
Doris wanted her to iron her yellow silk dress that she ‘must wear’ that night. She also wanted her mother to make tea for her. She refused to get her tea and iron her dress, telling her that she put in twice the hours Doris did but got neither wages, nor thanks for it.
Mother’s Day Questions And Answers Question 8.
What does Mrs Pearson say to Doris that really bothered her?
Answer:
Mrs Pearson asked where Doris would wear her yellow silk dress. She said that she planned to go out with Charlie Spence. Mrs Pearson told her to find somebody better, and insulted Charlie Spence by calling her buck-toothed and was half-witted.
Extra Questions Of Mother’s Day Class 11 Question 9.
What does Mrs Pearson have to say to Cyril that shocks him?
Answer:
When Cyril walk in and insists on her getting the tea and his clothes ready, he is stunned to hear that she doesn’t ‘like mending’. She goes on to tell him that when he does not want to do something, he does not do it. She planned to do the same. Cyril could not believe his ears.
Mother’s Day Class 11 Important Questions And Answers Question 10.
What do Doris and Cyril feel about Mrs Pearson’s changed behaviour?
Answer:
Doris and Cyril discuss that there is something wrong with their mother as she is not behaving in character. They discuss how Mrs Pearson behaved oddly with each of them. They try to fathom if she had gone crazy or had a concussion.
Mother’s Day Class 11 Questions And Answers Pdf Question 11.
What is Mrs Pearson’s reaction to see her children giggling when she returns to the room?
Answer:
Mrs Pearson asks them the reason for their amusement. Doris answers that she had never understood their jokes. To which Mrs Pearson retorts, rudely, that she was bored at their jokes even before they were bom. Doris is tearful and Mrs Pearson blames them for being selfish about their needs.
Mother’s Day Question Answer Question 12.
What reason does she give Cyril for not making the tea?
Answer:
When Cyril again asks for tea as he had been working for an eight-hour day, Mrs Pearson replies that she had done her eight hours and henceforth she would work only for forty hours a week. She declared that she would have her two days off on the weekend.
Important Questions Of Mother’s Day Class 11 Question 13.
What, according to Mrs Pearson, were her plans for the weekends?
Answer:
Mrs Pearson tells her children that at the weekend she would have her two days off. She agreed to make beds and cook a little as a favour, conditional to how she was treated. Mrs Pearson tells her children that in case they did not like the arrangement, she would go elsewhere for the weekend.
Mothers Day Class 11 Question Answer Question 14.
Why was George Pearson surprised when he came home? What was the answer that he got?
Answer:
Mr George Pearson was surprised to see Doris crying and was shocked to see Mrs Pearson sipping beer. He said that it did not look right. Mrs Pearson replied that it was ‘a nice change’ and it had been quite some time since he was surprised at her.
Extra Questions Of Chapter Mother’s Day Class 11 Question 15.
What did Mrs Pearson say to her husband when he was angry with her for not making tea?
Answer:
Mr Pearson informed Mrs Pearson that he did not want tea but grew angry at being told that tea was not ready. She taunted him that if he went up to the bar at the club and refused a glass of beer and showed irritation because they had not served it earlier, he would invite ridicule.
Question 16.
What was the truth about Mr George Pearson that hurt him the most?
Answer:
Mrs Pearson told George that that he was one of the standing jokes in the club. He was called ‘Pompy-ompy Pearson’ because they thought that he was slow and pompous. She was surprised that he spent so much time at a place where people always ridiculed him, leaving his wife at home.
Question 17.
What was Mrs Fitzgerald’s reaction to Mrs Pearson shouting at Cyril? Why?
Answer:
Mrs Fitzgerald was in reality Mrs Pearson, so when she saw Mrs Pearson (the real Mrs Fitzgerald) shouting at Cyril, she protested as she was actually Cyril’s mother. But Mrs Pearson told her not to interfere.
Question 18.
What were the two slips that could have let out the real identity of Mrs Fitzgerald?
Answer:
Mrs Fitzgerald, in her nervousness, addresses Mr Pearson, as George. Mr Pearson is surprised to be called thus, but Mrs Pearson covers up for Mrs Fitzgerald. Later, when Mrs Fitzgerald attempts to slap George, following an argument, the real Mrs Pearson exclaims and calls out to her, ‘Mrs Fitzgerald’, which confuses George.
Question 19.
How was the experience for the two women after the change of bodies?
Answer:
The real Mrs Pearson (now Mrs Fitzgerald) had not enjoyed the experience as she had seen her family being treated roughly and rudely by Mrs Fitzgerald. On the other hand, Mrs Fitzgerald had enjoyed the experience, as she had been able to teach Doris,Cyril and George Pearson a lesson to value Mrs Pearson.
Question 20.
What was Mrs Fitzgerald’s advice to Mrs Pearson after she had put back the family members in their proper place?
Answer:
Mrs Fitzgerald advised Mrs Pearson not to be soft and waste all the effort she had put in to change the attitude of her family for the better. Mrs Pearson is unsure how she would explain her behaviour. But Mrs Fitzgerald warns her not to yield.
Question 21.
What was the change that came over the Pearson family in the end?
Answer:
When Mrs Fitzgerald left, Mrs Pearson’s family was relieved to see her smile. Mrs Pearson decides to stay home for a family game of rummy, and have the children prepare dinner. They readily agree and gather around Mrs Pearson as the play ends.
Mother’s Day Extra Questions and Answers Long Answer Type
Question 1.
Write in your words the conversation between Mrs Pearson and Mrs Fitzgerald in the beginning of the play. What is the outcome of the meeting?
Answer:
Mrs Fitzgerald predicts her friend Mrs Pearson’s fate and tells her that it was high time she asserted herself as the head of the family. Mrs Pearson says that it was not easy because she loves her family although they are very thoughtless and selfish. But Mrs Fitzgerald insists that they ought to learn to appreciate her and treat her appropriately. She tells her not to run after them and oblige. Mrs Pearson agrees with Mrs Fitzgerald, but wonders if anything would affect them.
She is afraid of creating unpleasantness in the family. As Mrs Pearson is about to rush off to prepare dinner for her family, Mrs Fitzgerald comes up with an idea. She tells . Mrs Pearson that they could exchange their bodies. She then holds her hand and asks her to keep quiet for a minute. They stare at each other and Mrs Fitzgerald mumbles ‘Arshtatta dum—arshtatta lam—arshtatta lamdumbona…’ and they assume each other’s personality.
Question 2.
What does Mrs Pearson have to say to Doris that disturbs her?
Answer:
When Mrs Pearson’s daughter Doris returns and tells Mrs Fitzgerald, in the body of Mrs Pearson, to iron her yellow silk dress as she would like to wear it that night, her mother continues playing patience. Doris asks her what she is doing and she answers her smugly that she was not whitewashing the ceiling. She also says that there is no law against smoking. She informs Doris that she had already had her tea but had not made tea for the others.
She had not cooked dinner either and would have her meal at the Clarendon. She tells Doris that she worked twice as hard as the others and got no wages or thanks for it. She then inquired from Doris where she wanted to wear her yellow dress. Doris tells her that she was going out with Charlie Spence. Mrs Pearson tells her to find someone better than the buck-toothed and half-witted man. Doris is offended and runs out.
Question 3.
Describe Mrs Pearson’s conversation with Cyril when he walks in and his reaction.
Answer:
Mrs Pearson’s son Cyril walks in and insists on Mrs Pearson getting the tea and his clothes ready. He reminds her of the promise she made the same morning, to mend his clothes. He is surprised to hear that she does not Tike mending’ and that she would not do anything that she did not want to do. Cyril could not believe his ears. Cyril again asks for tea, telling her that he had been working for an eight-hour day, to which Mrs Pearson says that she had done her eight hours and henceforth she would work for only forty hours a week. On weekends, she would have her two days off.
She might make a bed or two and do a bit of cooking as a favour but that would be conditional to the fact that they asked her very nicely and thanked her for everything and generally made a fuss of her. Cyril and Doris are surprised and wait for their father to arrive.
Question 4.
“Sometimes it does people good to have their feelings hurt.” Who says this and what does she say to hurt Mr Pearson?
Answer:
When Mr Pearson announces that he did not wish to drink tea, after he returned from work, the real Mrs Fitzgerald informs him that his tea was not ready. He is angry and she reminds him that he was annoyed because he did not get the tea that he did not want in the first place. She adds that if he did that at bar—went up to the bar at the club and told them he did not want a glass of beer but got irritated because they had not already poured it out for him, they would laugh at him even more than they did already.
George was indignant and she added that he was one of their standing jokes and was called ‘Pompy-ompy Pearson’ because they thought that he was slow and pompous.
George checks with Cyril on the truth of the matter, and Cyril accuses his mother of not being fair and sensitive. To which, she replies that sometimes it does people good to have their feelings hurt.
Question 5.
Pick out the instances that bring out the element of humour in the play.
Answer:
The play is a light-hearted comedy. A mother accomplishes changing the attitude of her family by exchanging her body with her friend, Mrs Fitzgerald, a bold and assertive woman, thus, introducing humour in the play. The moment the spirits change, Mrs Pearson notices the cigarette, snatches it off Mrs Fitzgerald, while she looks down at her changed body and screams out of fright.
Mrs Pearson answers to her daughter’s query, sarcastically, that she was not whitewashing the ceiling. Mrs Pearson insults Doris’ date, comically, calling him ‘buck-toothed and half-witted’. Cyril is told that she does not ‘like mending’ his clothes, stunning him to silence. When Mrs Pearson walks out, Doris and Cyril laugh at the idea of her having gone crazy and decide to wait till the father comes. She tells Mr Pearson how he was a standing joke in the club and was called ‘Pompy-ompy Pearson’ because they thought that he was slow and pompous. She also tells him that he was George, and not the Duke of Edinburgh.
Question 6.
Mrs Fitzgerald’s effort does not go in vain, as the family changes for the better. Justify.
Answer:
Mrs Pearson has a selfish and a thoughtless family, who is insensitive to the feelings of the mother. But after the staged trick, and when Mrs Fitzgerald leaves, the three—George, Doris, and Cyril look anxiously at Mrs Pearson, who smiles. They are much relieved, and smile back at her. Mrs Pearson tells them that since they have decided to stay at home, they would have a nice family game of rummy and then the children could get the supper ready while she talked with their father. All of them agree. Mrs Pearson wishes Mrs Fitzgerald goodbye and the family surrounds Mrs Pearson, implying a happy ending. It looks as if Mrs Fitzgerald’s effort is rewarded and they seem eager to appease the real Mrs Pearson.