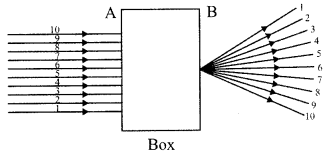
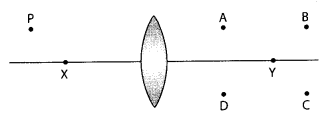
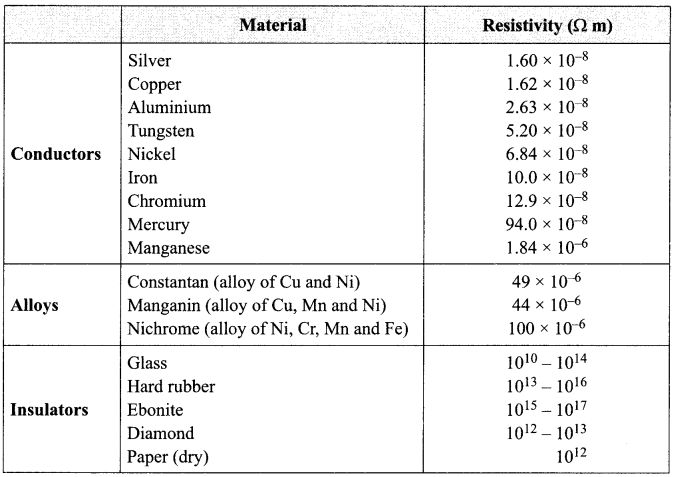
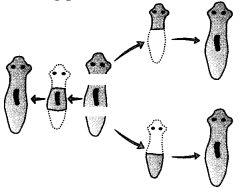
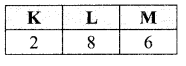
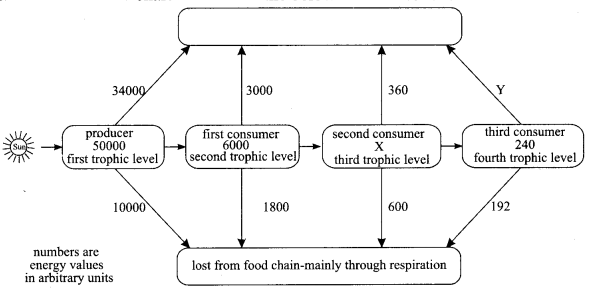
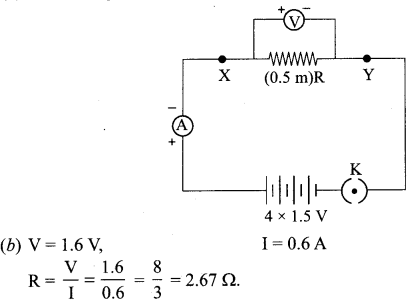
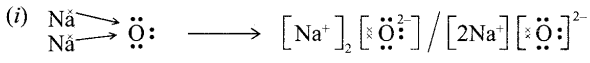Important Questions for Class 12 Physics Chapter Wise with Solution Pdf Download 2020-21: Here we are providing CBSE Important Extra Questions for Class 12 Physics Chapter Wise Pdf download in Hindi and English Medium. Students can get Class 12 Physics NCERT Solutions, Physics Class 12 Important Extra Questions and Answers designed by subject expert teachers.
CBSE Class 12th Physics Important Extra Questions and Answers Chapter Wise Pdf
- Class 12 Physics Chapter 1 Important Questions Electric Charges and Fields
- Class 12 Physics Chapter 2 Important Questions Electrostatic Potential and Capacitance
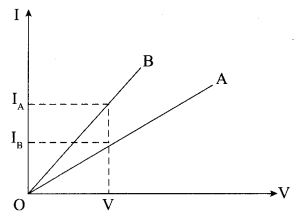
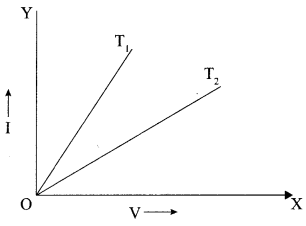
- Current Electricity Class 12 Important Questions
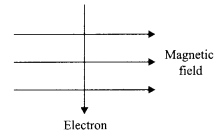
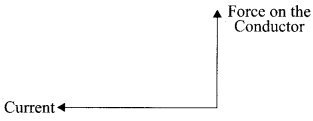
- Moving Charges and Magnetism Class 12 Physics Important Questions
- Magnetism and Matter Physics Class 12 Important Questions
- Electromagnetic Induction Imp Question of Physics Class 12
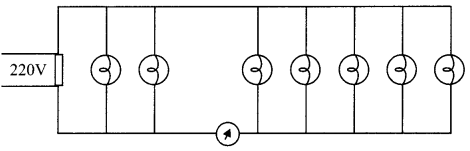
- Alternating Current Important Questions of Physics Class 12
- Electromagnetic Waves Physics Important Questions Class 12
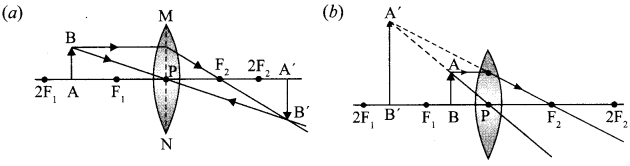
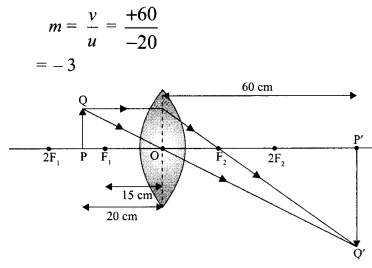
- Ray Optics and Optical Instruments Class 12 Important Questions
- Wave Optics Class 12 Important Questions
- Dual Nature of Radiation and Matter Class 12 Important Questions
- Atoms 12th Physics Important Questions
- Nuclei CBSE Class 12 Physics Important Questions
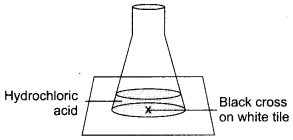
- Semiconductor Electronics: Materials, Devices and Simple Circuits Important Questions
- Communication Systems Class 12 Important Questions
We hope the given CBSE Important Questions of Physics Class 12 Chapter Wise Pdf download in Hindi and English Medium will help you. If you have any queries regarding NCERT Class 12 Physics Extra Important Questions and Answers, drop a comment below and we will get back to you at the earliest.