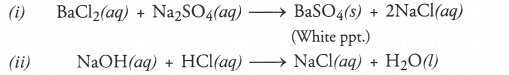
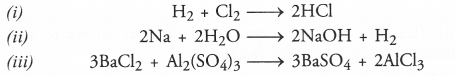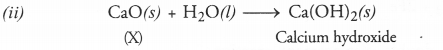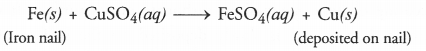Get Chapter Wise Online Education MCQ Questions for Class 7 Hindi with Answers Vasant Bhag 2 PDF Free Download prepared here according to the latest CBSE syllabus and NCERT curriculum. Students can practice CBSE Class 7 Hindi MCQs Multiple Choice Questions with Answers वसंत भाग 2 to score good marks in the examination.
Students can also visit the most accurate and elaborate NCERT Solutions for Class 7 Hindi. Every question of the textbook has been answered here.
Online Education Class 7 Hindi MCQs Multiple Choice Questions with Answers Vasant Bhag 2
Practicing these CBSE NCERT Objective MCQ Questions of Class 7 Hindi with Answers Vasant Bhag 2 Pdf will guide students to do a quick revision for all the concepts present in each chapter and prepare for final exams.
MCQ Questions for Class 7 Hindi Vasant with Answers
- हम पंछी उन्मुक्त गगन के Class 7 MCQ Questions
- दादी माँ Class 7 MCQ Questions
- हिमालय की बेटियाँ Class 7 MCQ Questions
- कठपुतली Class 7 MCQ Questions
- मीठाईवाला Class 7 MCQ Questions
- रक्त और हमारा शरीर Class 7 MCQ Questions
- पापा खो गए Class 7 MCQ Questions
- शाम एक किशान Class 7 MCQ Questions
- चिड़िया की बच्ची Class 7 MCQ Questions
- अपूर्व अनुभव Class 7 MCQ Questions
- रहीम की दोहे Class 7 MCQ Questions
- कंचा Class 7 MCQ Questions
- एक तिनका Class 7 MCQ Questions
- खानपान की बदलती तस्वीर Class 7 MCQ Questions
- नीलकंठ Class 7 MCQ Questions
- भोर और बरखा Class 7 MCQ Questions
- वीर कुवर सिंह Class 7 MCQ Questions
- संघर्ष के कराण मैं तुनुकमिज़ाज हो गया धनराज Class 7 MCQ Questions
- आश्रम का अनुमानित व्यय Class 7 MCQ Questions
- विप्लव गायन Class 7 MCQ Questions
- बाल महाभारत कथा Class 7 MCQ Questions
MCQ Questions for Class 7 Hindi Grammar with Answers व्याकरण
- अपठित गद्यांश Class 7 MCQ
- अपठित काव्यांश Class 7 MCQ
- वर्ण विचार Class 7 MCQ
- वर्तनी Class 7 MCQ
- संधि विचार Class 7 MCQ
- शब्द विचार Class 7 MCQ
- अनेकार्थी शब्द Class 7 MCQ
- पर्यायवाची शब्द Class 7 MCQ
- विलोम शब्द Class 7 MCQ
- वाक्यांश के लिए एक शब्द Class 7 MCQ
- तत्सम एवं तद्भव Class 7 MCQ
- उपसर्ग और प्रत्यय Class 7 MCQ
- समास Class 7 MCQ
- संज्ञा, कारक, सर्वनाम, विशेषण एवं क्रिया Class 7 MCQ
- मुहावरे एवं लोकोक्तियाँ Class 7 MCQ
We hope the given NCERT MCQ Questions for Class 7 Hindi with Answers Vasant Bhag 2 PDF Free Download will help you. If you have any queries regarding CBSE Class 7 Hindi MCQs Multiple Choice Questions with Answers वसंत भाग 2, drop a comment below and we will get back to you soon.